Ibrutinib is now approved in the USA for all indications for chronic lymphocytic leukemia. That hasn’t always been the case. Its first approval was more limited.
In fact, the first approval was not in CLL, but in its sister cancer with arguably an ever even greater need for more effective therapies, mantle cell lymphoma on Nov 13, 2013.
Not long after in terms of the FDA’s pace of action, but an eternity if you were a patient needing therapy, on February 12, 2014, the U.S. Food and Drug Administration (FDA) approved the use of Imbruvica (ibrutinib) for chronic lymphocytic leukemia (CLL) patients who have received at least one previous therapy. That was a pretty broad approval. It didn’t say that the patient had to have failed a prior therapy, just tried one.
This quickly resulted in the gaming of the system where patients might take a few days of chlorambucil or might just say that they took it without ever actually swallowing a pill. They then reported to their hematologist that the oral chemo pill made them nauseated and asked if they could they now move on to ibrutinib, which would then be approved.
On July 28, 2014, the U.S. Food and Drug Administration (FDA) also approved Imbruvica for all CLL patients with del 17p, a genetic mutation that occurs when part of chromosome 17 has been lost, regardless of whether they had used a prior therapy or not. As we know, CLL patients with del 17p are considered to have the poorest prognosis and ibrutinib markedly improves that prognosis.
On March 4, 2016 the U.S. Food and Drug Administration (FDA) finally approved Imbruvica (ibrutinib) as a first-line treatment for patients with chronic lymphocytic leukemia (CLL). That approval was based on data from the RESONATE-2 trial, which evaluated the use of Imbruvica versus chlorambucil in 269 treatment-naïve patients with CLL or small lymphocytic lymphoma (SLL) aged 65 years or older.
Today, essentially any CLL / SLL patient in the USA can take ibrutinib. The need to actually take another therapy first or at least to say you did is no longer in play.
On May 9, 2016 the U.S. Food and Drug Administration (FDA) updated the Imbruvica (ibrutinib) Prescribing Information (PI) to include new data from two Phase 3 trials supporting its expanded use in patients with chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL) The label includes the improved overall survival (OS) results in previously-untreated CLL / SLL patients from the RESONATE 2 trial.
The Canadian approval for frontline use came four months after the US approval on July 20, 2016 when Health Canada approved Imbruvica (ibrutinib) for previously untreated patients with active chronic lymphocytic leukemia (CLL). This was the 4th approval for ibrutinib in Canada following a similar pattern as in the USA. Since July 2016, it has been approved for use in all lines of CLL therapy for patients needing treatment offering Canadian patients a chemotherapy-free option.
However, approval does not mean that the medication is paid for. In Canada, a drug may be approved, but not covered by the local provincial formulary, rendering it inaccessible for most.
Here is an article I wrote for the opinion section of the Washington Times in 2010 when I was dependent on another drug, rituximab, to control my aggressive ITP (immune thrombocytopenic purpura).
https://www.washingtontimes.com/news/2010/mar/17/a-prescription-for-life-or-death/
Australian patients face similar challenges where federal approval doesn’t guarantee payment.
There are many other ways governments can restrict access to a drug. Such a situation was just resolved this week in the United Kingdom.
And these regulatory issues don’t touch on the cost of the medications not covered by insurance, whether that insurance is supplied by the government (i.e. Medicare in the USA) or private companies.
Why is all this so important?
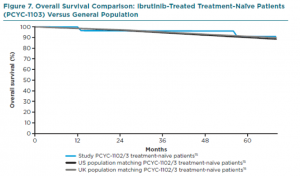
Here is a graph comparing survival of CLL patients on ibrutinib frontline versus matched controls from the US or UK. In other words, comparing CLL patients on ibrutinib with normal controls.
It looks as if having CLL that needs treatment and getting ibrutinib as your first therapy gives you the same life expectancy as someone without CLL, at least for the first 5 years. That’s as far out as we have data.
Take a long look and then let the importance sink in. There is a good reason that ibrutinib is approved for the treatment-naïve patient and it should be high on the list of everyone who is in need of therapy.
While a significant percentage of patients with good prognostics (mutated IGVH and no adverse FISH findings) can live more than 15 years with no progression of their chronic lymphocytic leukemia after only 6 months or less of the state of the art chemo-immunotherapy, FCR, eliminating the need for long term treatment, this ibrutinib graph is for all comers, not just a percentage of those with less aggressive disease.
What a long way we have come!
Stay strong. We are all in this together.
Brian Koffman, MD
8/13/18