This is a synopsis of the research we presented at the 23rd Congress of the European Hematology Association, Stockholm, Sweden. June 14-17, 2018.
The link to the poster is here.
Our presentation at ASCO 2018 was very similar. Here’s a link to that poster.
For those who wish to read the abstract, here is the link to the ASCO abstract.
I am very proud of this research. It is the largest survey of its kind ever done on CLL patients and would not have been possible without 4 factors:
- The willingness of 1147 patients to complete a 64 question survey. I am in their debt.
- The recruitment efforts by the “who’s who” in CLL.
- The hard work of the Dr. A. Mato, B. Dennison, K. Kennard and yours truly on the abstract and poster.
- Our partners and sponsors.
Here is an overview. I know it’s a lot to read and this is just one part of the research. We hope to present more at ASH 2018 and publish the work in a peer reviewed journal.
If you are overwhelmed, just skip the diagnosis section of the results and read forward from there.
I will make some comments about this research and why I think it’s so important over the next weeks, but for now, please take your time reviewing the data we presented at EHA and ASCO, then please share your comments and feedback via https://cllsociety.org/contact-us/.
A US-Based Survey: The Experiences of 1147 CLL Patients
Koffman1, B. Dennison2, K. Kennard3, R. Furman4, J. Byrd5, J. Pagel6, M. Davids7, C. Nabhan8, N. Kay9, T. Siddiqi10, D. Brander11, W. Wierda12, D. Stephens13, B. Hill14, J. Pinilla-Ibarz15, B. Cheson16, S. Rosen10, F. Awan5, M. Choi17, A. Mato18
1CLL Society Inc., Claremont, CA; 2CLL Society Inc., Pompton Lakes, NJ; 3Abramson Cancer Center, Philadelphia, PA; 4Weill Cornell Medicine, New York, NY, 5The Ohio State University, Division of Hematology, Columbus, OH; 6Swedish Cancer Institute, Seattle, WA; 7Dana-Farber Cancer Institute/Harvard Medical School, Boston, MA; 8Cardinal Health Specialty Solutions, Dublin, OH; 9Mayo Clinic, Rochester, MN; 10City of Hope National Medical, Duarte, CA; 11Duke University School of Medicine, Durham, NC; 12MD Anderson Cancer Center, Houston, TX, 13University of Utah Huntsman Cancer Institute, Salt Lake City, UT; 14Cleveland Clinic Foundation, Cleveland, OH; 15H. Lee Moffitt Cancer Center & Research Institute, Tampa, FL; 16Georgetown University Hospital, Lombardi Comprehensive Cancer Center, Washington, DC; 17Moores Cancer Center, University of California San Diego School of Medicine, San Diego, CA; 18Memorial Sloan Kettering Cancer Center/CLL Program, Leukemia Service, New York, NY
PS1107 presented at the 23rd Congress of the European Hematology Association. Stockholm, Sweden. June 14-17, 2018
CLL in 2018
The CLL literature focuses largely on the objective aspects of diagnosis, active observation (AO), prognosis and management. While health care providers (HCPs) try to effectively educate and reassure patients, the literature on patients’ subjective experience through the continuum of care is limited.
Introduction
The CLL Society Inc., a patient-driven, physician-curated nonprofit organization focusing on the unmet needs of the CLL community, sought to understand how CLL and its management, including the healthcare team, diagnosis, education, active observation, prognostic testing, and the options of clinical trial affect the lives of those with CLL and what was important to patients. The goal was to better understand CLL from the patient’s perspective to inform HCPs in best meeting their patients’ needs.
Objectives
- To assess patient understanding of CLL in terms of symptoms (physical and emotional), diagnosis, prognostic testing to identify specific areas for education and support.
- To assess the experiences of patients in “Watch and Wait / active observation” including psychosocial stresses such as anxiety, depression and fatigue, to identify areas for education and support.
- To assess patient experience related to the option of a clinical trial.
Study Design
The 2017 CLL Diagnosis and Treatment Assessment: The Patient View (2017 CLLDATA) survey included 64 multi-part questions directed to CLL pts to capture more information on their experience with CLL. The survey, which was available online or on paper, was IRB-approved and took place between October-December 2017. This report will focus on the patient perspective at diagnosis, during the active observation period, prognostic testing, clinical trial participation and sources of information. Data on other topics within the survey will be reported in the future.
Inclusion criteria
Patients must be age 18 or above, have a diagnosis of CLL / SLL, be a United States resident with a working knowledge of English and be able to provide informed on-line or paper consent for study participation.
Survey Recruitment
This survey was administered anonymously using both the CLL Society (CLLS) database, Facebook page, bkoffman.blogspot.com, and online CLL patient forums, as well as information cards distributed in the offices of the principal investigators (PIs) and identified physicians that refer to those PIs, as well as attendees at CLL Society patient support groups. A self-selected convenience sample resulted from online methods, as well as informational cards provided to CLL patients within the offices described above, and CLL Society Patient Support Groups. Patients could participate via an online or a paper survey.
Statistical Analysis
Data were analyzed using descriptive methods. Chi-square was used to evaluate statistical significance. Analyses and comparisons were made between the multiple subgroups made between the multiple subgroups
- <65 year old patients versus >65 year old patients
- Untreated versus treated patients
- Male versus female
- High-risk versus low-risk
- Patients treated by a general hematologist/oncologist only, a CLL expert only, or both
Definitions
For the purposes of this research, we used the following definitions:
- General hematologist/oncologist – Sees all types of cancer patients
- CLL Expert – A hematologist/oncologist who primarily focuses on CLL and works at an academic or research center
- High-risk – 17p del, 11q del, unmutated IGHV
- Low-risk – Trisomy 12, 13q, IGHV mutated with no 17p del or 11q del
Results
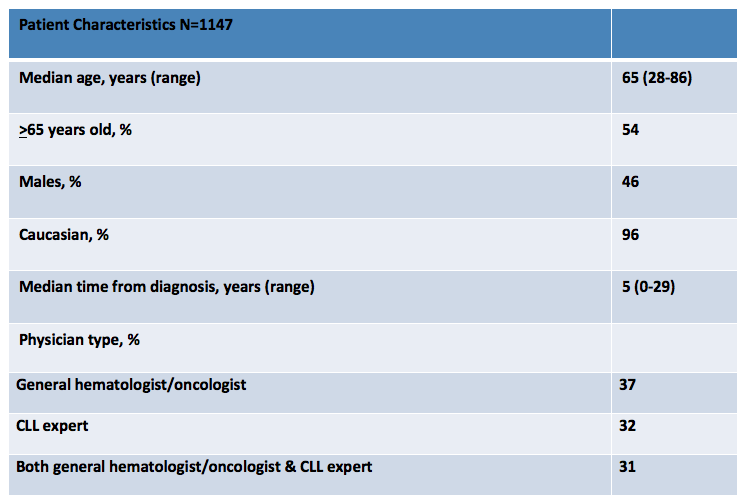
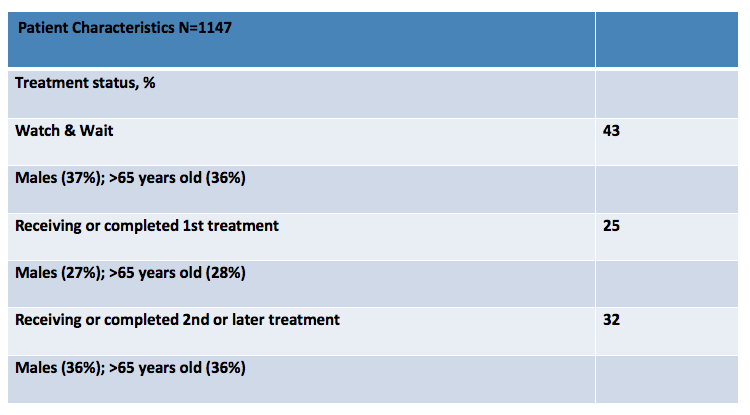
Diagnosis
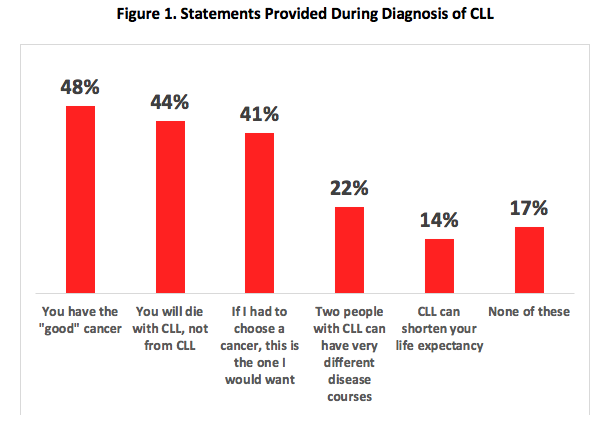
At diagnosis, 48% of patients were told they had the “good” cancer by their HCP. This statement, as well as others were reported equally by patients regardless of the type of physician managing/treating their CLL. [Figure 1]
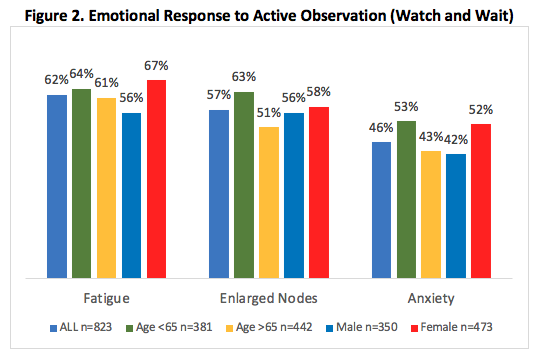
Only 13% of patients reported needing treatment within 3 months of diagnosis. For those managed with active observation (87%), 91% stated that their doctor explained why therapy was not warranted. However, active observation the majority of patients reported anxiety (56%), relief (52%) and confusion (31%). [Figure 2.]
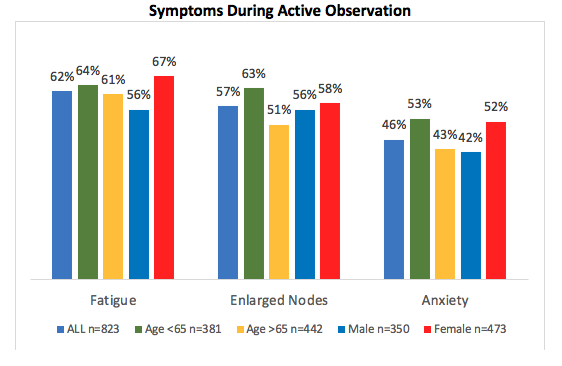
Use of Alternative Therapies During Active Observation
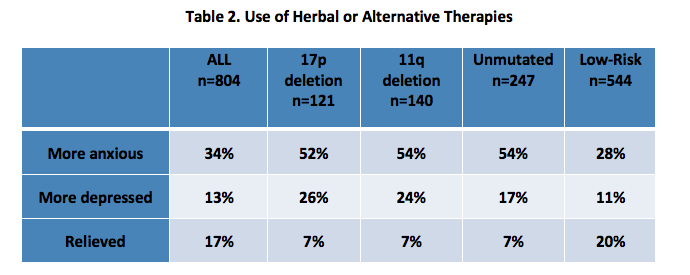
Respondents were asked to report on their use of herbal and other non-traditional interventions for managing their CLL during the active observation period, and 66% reported doing so. (Table 2). Fifty-nine percent of patients aged 65 or older reported use of herbal or alternative therapies compared to 75% of patients younger than 65 (p<.001). There was no difference in use of specific herbal or alternative therapies between age groups. Between genders, utilization of herbal or alternative therapies was equal with the exception that women more often use Vitamin D and prayer.
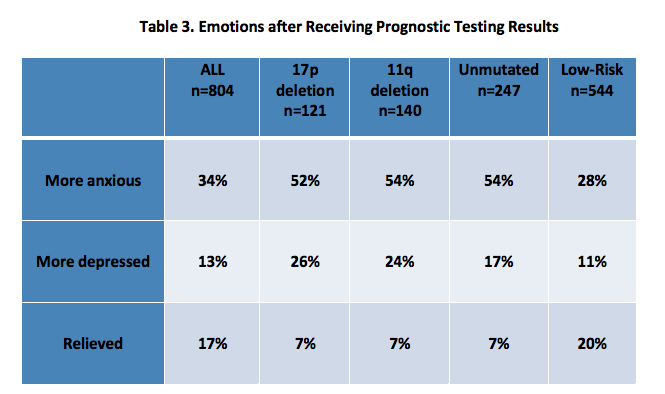
 Ninety-two percent of respondents report having had some type of prognostic testing, although 9% were not aware of what specific testing was performed. Two-thirds reported testing at the time, or soon after diagnosis, 33% prior to their first treatments and 7% before 2nd or later treatment. Twenty percent of those tested were unaware of their test results, 12% reported 17p deletion and 14% reported 11q deletion. Respondents with high-risk prognostic markers (17p, 11q, unmutated) reported higher levels of anxiety and depression, compared to those with low-risk prognostic markers (Trisomy 12, 13q, IGHV mutated with no 17p deletion or 11q deletion). [Table 3]
Ninety-two percent of respondents report having had some type of prognostic testing, although 9% were not aware of what specific testing was performed. Two-thirds reported testing at the time, or soon after diagnosis, 33% prior to their first treatments and 7% before 2nd or later treatment. Twenty percent of those tested were unaware of their test results, 12% reported 17p deletion and 14% reported 11q deletion. Respondents with high-risk prognostic markers (17p, 11q, unmutated) reported higher levels of anxiety and depression, compared to those with low-risk prognostic markers (Trisomy 12, 13q, IGHV mutated with no 17p deletion or 11q deletion). [Table 3]
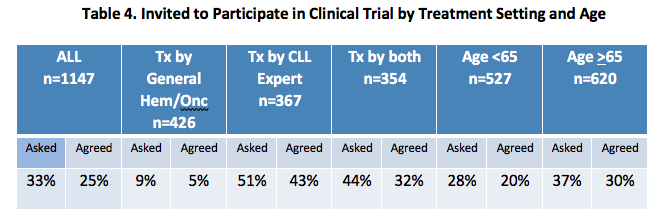
Clinical Trial Considerations
Thirty-three percent of respondents reported being invited to participate in a clinical trial at least once. Over half of patients being treated by a CLL expert reported being invited to participate in a clinical trial, and 43% agreed to participate, compared to 5% of those treated in the community setting. [Table 4]
Respondents that were invited but declined participation in a clinical trial, or reported that they would decline participation if asked, reported reasons such as:
- Preference for a “proven” treatment – 38%,
- Distance from the trial site – 29%,
- Fear – 20%
- Frequent imaging – 20%.
Respondents that agreed to participate in a clinical trial cited the following reasons:
- My healthcare team suggested a trial as a good treatment option for me – 71%
- Access to the latest treatments – 69%
- Close monitoring of my CLL – 67%
- I felt confidence in the trial team – 65%
- I wanted to help others – 56%
- Medications provided for free – 54%
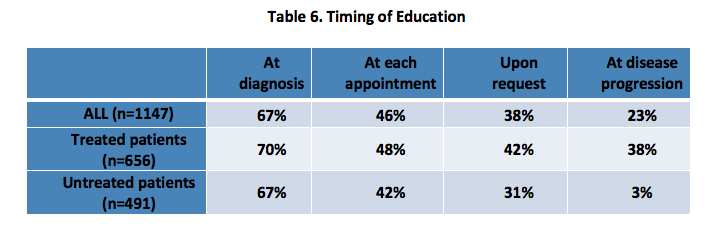
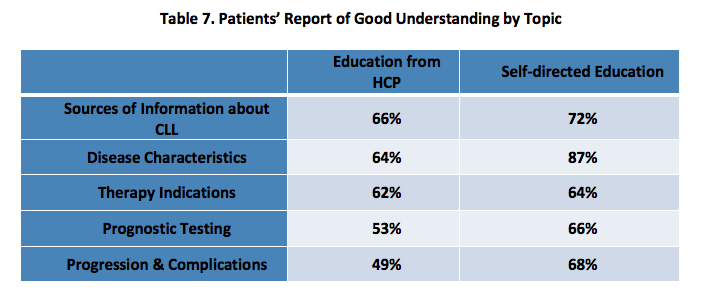
Respondents were asked to report about the format in which they received education from their healthcare team, when they received this education, their level of understanding after receiving education, whether they sought information for themselves and its impact. [Tables 5-7]
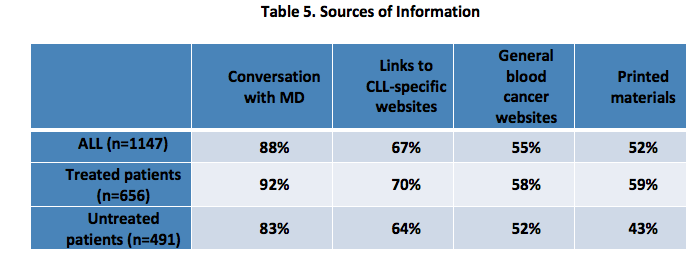
Ninety-eight percent of respondents reported looking for additional information after education received from their healthcare team, most often using an online resource, specifically a CLL-specific website (90%), online CLL patient blog or forum (69%) and general blood cancer websites (64%).
Conclusions
To our knowledge, this is the largest survey of CLL patients. Much can be learned by detailed surveying of CLL patients throughout their disease. These include previously unrecognized suboptimal interactions between the CLL patient and the HCP. Understanding how patients experience their disease is critical to improve communication between patients and their HCPs, which will ultimately advance CLL outcomes.
Recommendations
- 72% of patients reported being offered language similar to “CLL is the good cancer” at the time of diagnosis. This might be better replaced with the facts that CLL is a variable disease, with some patients never needing treatment and others experiencing more aggressive disease.
- AO is an active and symptomatic time for patients with 2/3 using alternative therapies. Patients need more support during active observation.
- Prognostic testing led to increased anxiety in 1/3 of patients, including in 28% who had low-risk disease suggesting a need for improved education.
- Only 7% of patients recall prognostic testing before 2nd or later therapy indicating an unmet need to ensure both patients and providers understand the importance of prognostics when re-treating.
Limitations
Our patient respondents were younger (median age 65 years old) compared to the median age of 70 in SEER data2. They were also more female (54%) than generally reported (38%)1. This likely reflects a selection bias of those completing the survey.
Thank You
The CLL Society wishes to thank the patients who participated in this important research and the physicians and support group facilitators that helped create awareness.
















