Over the last 8 years helped lead the charge of new targeted therapies in chronic lymphocytic leukemia with the approval of ibrutinib.
Ibrutinib is the first in class FDA approved oral targeted therapy that blocks the B cell receptor (BCR) by tightly binding downstream to Bruton’s Tyrosine Kinase (BTK) and preventing it from sending the pro- survival signaling to the cancer cell and changing CLL treatment forever.
But ibrutinib inhibits other kinases. Kinases are simply enzymes that are involved in metabolism, cell signaling, protein regulation and cellular transport.
The other kinases inhibited by ibrutinib may lead to some of its “off target” side effects but might also contribute to its efficacy by its effects on the T cells and other “nurse- like” cells that support the CLL’s growth and longevity.
Acalabrutinib is a more specific BTK inhibitor approved for use in a related B-cell cancer, mantle cell lymphoma (MCL) but not CLL, although it is recommended in the NCCN guidelines for those intolerant of Ibrutinib. That’s good as it helps ensure that insurance will cover it.
There are ongoing studies that will likely lead to its approval in CLL.
I interviewed Dr. Byrd about an acalabrutinib trial that he led and presented at ASH 2018 in San Diego.
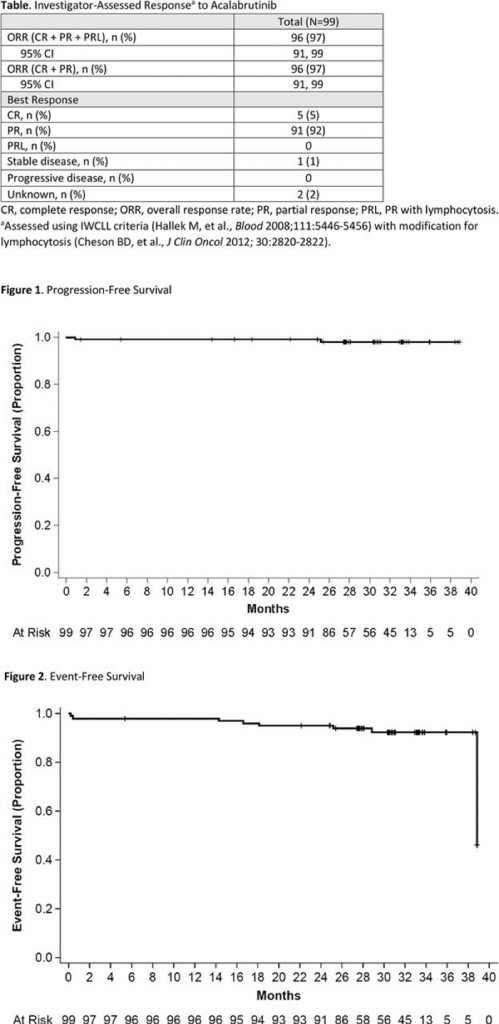
- In 99 treatment naïve chronic lymphocytic leukemia patients, there was a 97% overall response rate (ORR).
- The responses are durable over the 44 months that the patients have been followed (see Figure 1 and 2 below with the nearly flat line survival curves showing very few patients falling below the 100% line by progressing or by having an “event” such as death, progression or stopping the drug due to adverse events (AEs).
- Only 2 went off acalabrutinib due to disease progression.
- Infections tended to occur early and there was one fatal infection.
- Common problems were diarrhea (47%) and headaches (44%) with the latter seeming to be a specific side effect with acalabrutinib that is usually easily handled with acetaminophen.
- Bleeding and bruising rates are similar to ibrutinib.
- There may be less atrial fibrillation and hypertension.
- The numbers are small but so far there have been no cases of Richter’s Transformation.
- A study comparing acalabrutinib, acalabrutinib with obinutuzumab, and chlorambucil with obinutuzumab may lead to its approval, but it is taking a long time to read out as all arms are doing so well.
Conclusions:
Acalabrutinib is a second generation BTK inhibitor that appears to be very active in CLL and may have a different and milder side effect profile than ibrutinib.
Here is our interview from the last day of ASH 2018
Here is the link to the abstract itself