Patients with CLL / SLL rarely reach complete remission (CR) or undetectable measurable or minimal residual disease (U-MRD) with the oral BTK inhibitor ibrutinib. Treatment is expected to continue indefinitely. Combining a second agent, oral venetoclax, which works by a different pathway than Ibr, offers a potentially synergistic benefit and does so with an acceptable rate of adverse events (AE). Prior studies have shown this to be a safe and effective combination. The hope is that U-MRD can be achieved after a finite treatment period by using these two oral agents together. During the Covid pandemic, this combination also offers the benefit of avoiding the immune suppression caused by the CD20 targeted monoclonal antibody, obinutuzumab, which is currently used in combination with venetoclax finite duration treatment.
At the annual meeting of the American Society of Hematology (ASH) 2021, Dr. Mathew Davids from the Dana-Farber Cancer Institute in Boston interviews Dr. Bill Wierda from the MD Anderson Cancer Center in Houston. They discuss the benefits of combined finite therapy to achieve U-MRD with a tolerable level of adverse events (AE).
Takeaways:
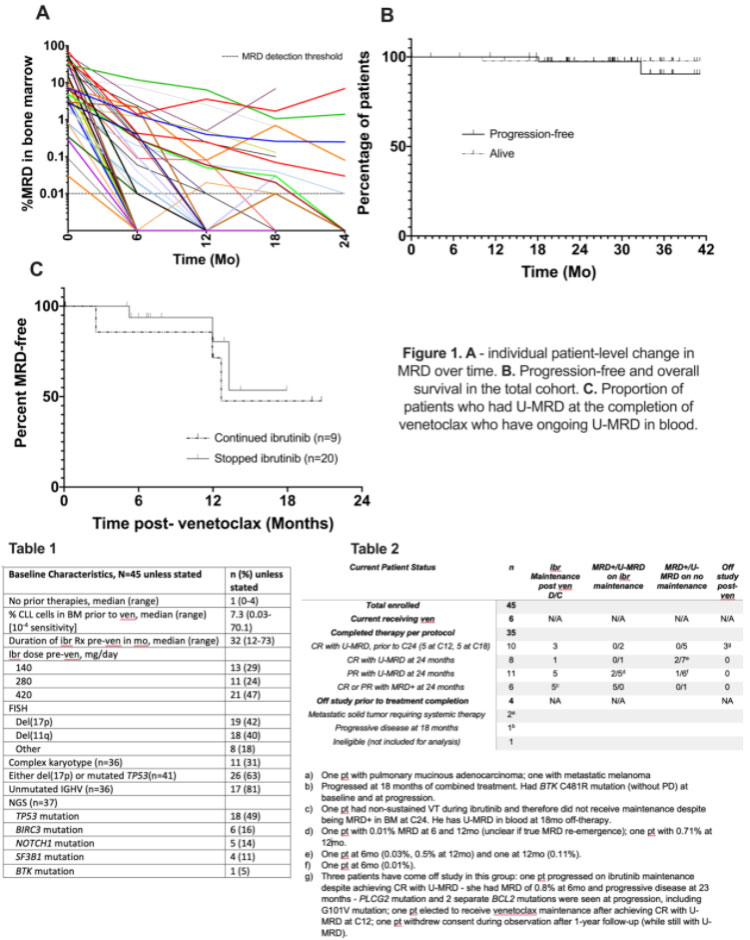
- All 45 patients evaluated in this study had at least one high-risk factor for progressions such as complex karyotypes, del(17p), del (11q), or persistent elevation of elevated B2 microglobulin. In addition, they had residual disease and had been on ibrutinib for at least one year before the addition of venetoclax.
- The combination therapy was extended for a maximum of 24 months.
- All patients had bone marrow (BM) evaluation by flow cytometry and CT scan for restaging every six months. Those patients with U-MRD on two consecutive evaluations six months apart stopped venetoclax. After 24 months of combined therapy, those patients who did not achieve U-MRD continued ibrutinib maintenance.
- After 12 months of combined therapy, 57% achieved U-MRD.
- At a median follow-up of 29 months, 29 of the 35 CLL patients that completed the treatment protocol achieved U-MRD.
- Of the 29 patients with U-MRD, 5 stopped therapy at cycle 15, and an additional five stopped at cycle 18.
- After completing venetoclax, 9 of 29 CLL patients with U-MRD continued Ibr at physician discretion.
- Two patients progressed, one during venetoclax plus ibrutinib and one at 23 months after venetoclax plus ibrutinib during ibrutinib maintenance.
- Please see the attached tables for more detail.
- The most common AE was grade 1-2 or mild diarrhea in 61% of patients, while 22% developed grade 3-4 or severely decreased neutrophils. There were no febrile cases of neutropenia, while there were three patients with grade 3 or moderate infections.
Conclusions:
After a median of 29 months, 35 patients with high-risk factors for progression completed the combination oral therapy of ibrutinib plus venetoclax. This treatment combination was well tolerated and produced U-MRD by bone marrow evaluation in 73% of high-risk patients. Many of these patients were able to stop all treatment and maintain U-MRD. However, only two patients progressed, one during the combined therapy and one at 23 months after completing combined therapy plus Ibr maintenance.
Unanswered questions include:
- Would the addition of a monoclonal antibody such as obinutuzumab increase the rate of U-MRD?
- If this therapy works so well in high-risk patients, might it even be curative in some low-risk patients with mutated IgVH, as we may have seen with FCR in the chemotherapy era?
These results provide hope for a combined therapy using the surrogate marker, U-MRD, to tell us we can stop therapy and enjoy a disease-free, side-effect-free period off CLL / SLL drug therapies.
It also suggests that it may make sense to intervene with more therapy to deepen response instead of waiting for clinical relapse. If that proves to be a successful strategy, that will upend much CLL / SLL care
Please enjoy the video interview with Dr. Wierda from the ASH 2021 meeting.
You can read the abstract here: Venetoclax Consolidation in Patients with High-Risk CLL Who Have Been on Ibrutinib More Than a Year Achieves a High Rate of Undetectable Minimal Residual Disease.
Be strong. We are all in this together.
Dr. Michael R. Green MD and CLL patient.