By Brian Koffman, MD
There are many prognostic and predictive tests that can be ordered at different times on our CLL journey.
Let’s start by saying that there is no consensus on this issue and different doctors have different opinions. I am sharing mine.
Here are my own thoughts on this frequently asked question.
This only applies if the correct diagnosis of chronic lymphocytic leukemia has first been locked in by flow cytometry (FC). Dr. Brown discusses the diagnosis issues here. With one minor exception (CD38), FC is less important for predictive and prognostic testing.
I will cover the following topics:
- Essential prognostic versus predictive test
- Statistics and their pitfalls
- Explain the significance of the tests
- My opinion on which tests, when and why
Prognostic versus Predictive Markers:
Prognostic markers suggest, for a group of individuals, the odds that their disease will be slow moving (indolent) or more rapid and aggressive.
Predictive markers predict, for a group, which therapies are likely to have good deep, responses.
Many biomarkers have both predictive and prognostic value.
For more on predictive and prognostic factors, please see my 2016 interview with Dr. Stephan Stilgenbauer.
Statistics and the Pitfalls
First, for anyone who reads any statistical analysis, I strongly recommend reading this short classic by the late great science writer, Stephen Jay Gould: The Median isn’t the Message. This is mandatory reading!
Next, Dr. Rick Furman reminds us of Arthur Conan Doyle’s quote from his book, The Sign of the Four:
“Winwood Reade is good upon the subject, said Holmes. He remarks that, while the individual man is an insoluble puzzle, in the aggregate he becomes a mathematical certainty. You can, for example, never foretell what any one man will do, but you can say with precision what an average number will be up to. Individuals vary, but percentages remain constant. So says the statistician.”
Despite Winwood Reade’s admonition, it would be a huge mistake for any individual who had 17p deletion or TP53 mutation or unmutated immunoglobulin heavy-chain variable region (IGHV) gene to ever consider chemoimmunotherapy.
With either of the first two markers, chemo-immunotherapy simply won’t work. With the third, chances are very small that one would get a durable remission.
The Biology of the Tests:
IGVH Somatic Hypermutation Status:
The purpose of B cells is to recognize foreign proteins. They are schooled in this inside lymph nodes where they mature and become “mutated” or rearranged to recognize a specific threat. When the cancerous CLL clone arises from the more mature “mutated” B cells, the cells tend to grow much more slowly, thus being “prognostic” of a more indolent disease course. To confuse matters, IGVH is also called IGHV. Both are considered correct.
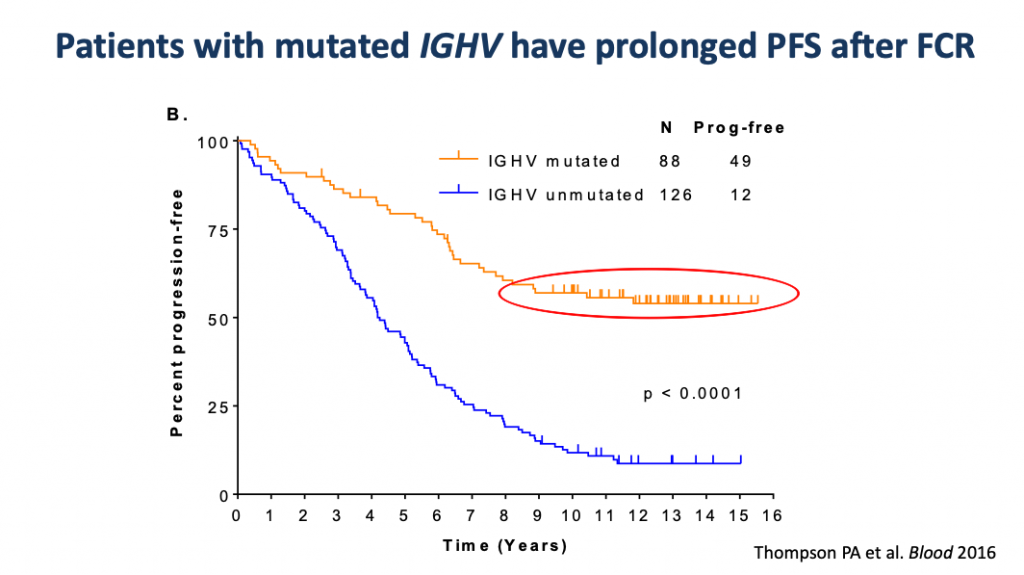
It is also “predictive” of a shot at a very durable response to FCR chemo-immunotherapy. The part of the Kaplan-Meier chart (below) in the red oval represents approximately 55% of the mutated patients who were given FCR and whose disease has not progressed out to 16 years after treatment. The plateauing of the PFS curve (progression free survival) hints at a possible…. cure.
If you want a deeper understanding of the Kaplan-Meier curves that are so ubiquitous in cancer literature, this NIH (National Institute of Health) article will help.
Essentially on this one, the X or horizontal axis is time in years, and the Y or vertical axis is the percentage of patients that have not progressed. Here we see that almost no one progresses if they make it to 9 years without the CLL coming back.
Therefore, IGHV mutation testing MUST be done before considering FCR therapy. With ibrutinib therapy, there is no difference in the outcome in mutated or unmutated patients, so the test is not necessary.
Unlike other tests that can change over time, IGHV never changes, so only needs to be tested once.
FISH (fluorescent in situ hybridization):
FISH looks for specific common chromosome abnormalities seen in CLL. The most important of these is a missing a piece of the short (p) arm of the 17th chromosome, known as 17p deletion. It generally probes for t(11;14); t(11q;); +12; del(11q); del(13q); del(17p).
This is important because the TP53 gene is found on the short arm of the 17th chromosome. TP53’s mission is to fix the damaged DNA, and if it can’t correct the injury, then it will kill off the “beyond repair” cell.
Chemo-immunotherapy works by damaging the DNA. That damage should signal the cell to begin the process of apoptosis or programed cell death. Chemo-immunotherapy can only be effective if there is an intact TP53. Without TP53, the damaged cells just keep going, potentially making things even worse.
TP53 Mutation Analysis:
We know that TP53 is important. TP53 has been called the guardian of the genome because when it is functioning well, it protects our genetic information from being miscopied and thus spawning a malignant offspring. When it is missing or dysfunctional, cancers are both meaner and harder to kill. Here is a nice overview on TP53 from the NIH. We want “wild type” TP53. That means the TP53 is in its normal functional “wild” state.
Sadly, TP53 is mutated and non-functional in many CLL patients who do not have a 17p deletion. So, for the same reasons that we do a FISH test, we need to ask our doctor to order a TP53 mutational analysis. This is rarely done outside of a CLL specialist’s office
Besides being resistant to chemotherapy, a person with a CLL clone that is 17p deleted or harbors a TP53 mutation is somewhat less responsive to ibrutinib, especially in the relapsed setting.
Conventional Karyotype Analysis:
This is a more “old school” examination of all 23 chromosomes. It is a technically difficult test to perform but tells us of all the less common abnormalities, not just the specific changes probed by FISH. We know that patients having CLL with multiple chromosomal abnormalities, called a complex karyotype, (CK) carry a less stable form that is more likely to mutate and become resistant to any therapy. This is true for both CIT and ibrutinib.
It makes sense to have these last three tests in all cases before treatment, because they may rule out chemo and suggest that it might make more sense to enroll in a clinical trial or use a combination of targeted therapies.
Next Generation Sequencing (NGS):
NGS is becoming more important in understanding CLL. It is testing that usually allows one to examine an array of genetic information from the cancer cell’s DNA and RNA. This can inform us as to what treatments might work. Except for TP53 and perhaps Notch1, this information is less important in CLL than in other cancers at this time. It may also predict how aggressive the cancer might be. Sometimes it can suggest possible new treatment options based on “actionable” genetic mutations. It is part of many clinical trials, but with the important exception of testing for TP53, it is still not used much clinically in CLL. That may be changing.
When to Test?
The obvious answer that screams out is “TEST BEFORE TREAT™. This is mandatory! Every time, before each and every treatment!
What is less certain is the advisability of testing at time of diagnosis.
This should be a decision made between doctor and patient. Some folks, despite my admonition concerning statistics, want to know if they are a “high-risk” patient so they can best prepare. I fully respect that decision and I fall into that group. Others would prefer not to know and that, too, is a respectable decision.
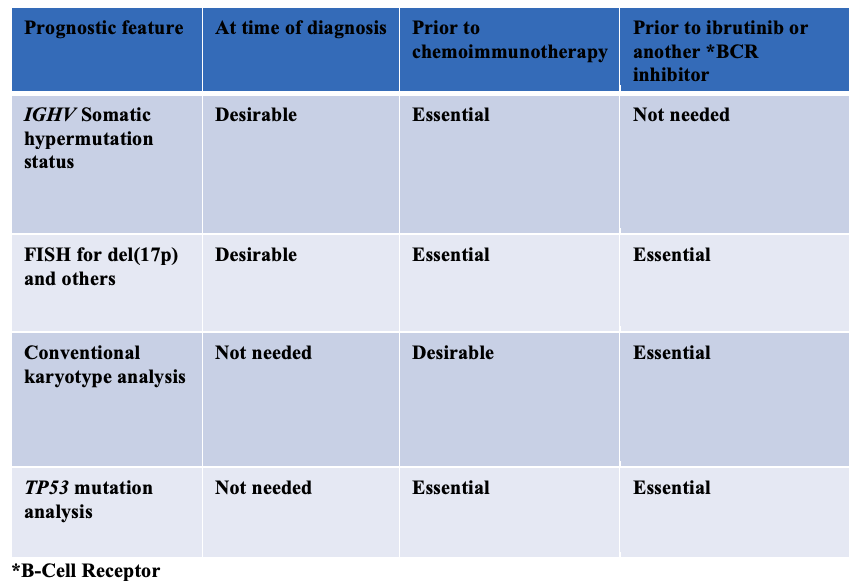
Here is a table for some of the most common decision points re: testing.
What Test to Order and When (with thanks to Dr. Thompson)
The table is missing decisions about other treatment options such as venetoclax or a clinical trial.
It is also leaving out a whole lot of next gen sequencing tests (see here for one of many articles and interviews on this topic) such as NOTCH1 (which may predict lack of response to rituximab- see here and here), SF3B1, XOP1, and others such as B2M, CD38 and ZAP 70 (see here).
But it’s a start and a simple minimum standard to ensure we are all getting the testing that we need.
And remember to always:
TEST BEFORE TREAT™
Dr. Brian Koffman, a well-known retired doctor, educator, and clinical professor turned patient has dedicated himself to teaching and helping the CLL community since his diagnosis in 2005. He serves as the Executive Vice President and Chief Medical Officer of the CLL Society Inc.
Originally published in The CLL Tribune Q3 2019.