INTRODUCTION:
Ibrutinib (Ibr) is the first approved covalent or irreversible binding Bruton Tyrosine Kinase inhibitor (BTKi). While this oral medication has dramatically improved the treatment of chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL / SLL), there is concern about the risk of heart or cardiovascular (CV) adverse effects due to off-target kinase inhibition. These adverse events (AE) include high blood pressure or hypertension (HT), racing heart conditions called atrial fibrillation (AF) or ventricular tachyarrhythmias (VTA’s), and sudden death.
The current phase III study, FLAIR, was performed by multiple researchers at multiple facilities and evaluated the possible connection between Ibr and sudden cardiac death.
Takeaways:
- Various previous trials identified a potential risk of cardiovascular AE with the use of Ibr treatment regimens. For example, in the RESONATE-2 Trial, there were nine unexplained or cardiac deaths; in the Alliance Trial, there were 11 deaths; in the ECOG1912 Trial, there was one death; and in the HELIOS trial, there were three unexplained and five heart-related deaths.
- In the current phase III multicenter, randomized FLAIR study, 771 previously untreated patients aged less than or equal to 75 years who needed treatment were recruited. They were deemed fit for fludarabine/cyclophosphamide/rituximab (FCR) and then randomized to receive either FCR or Ibr plus rituximab (IR).
- Patients with unstable angina were excluded.
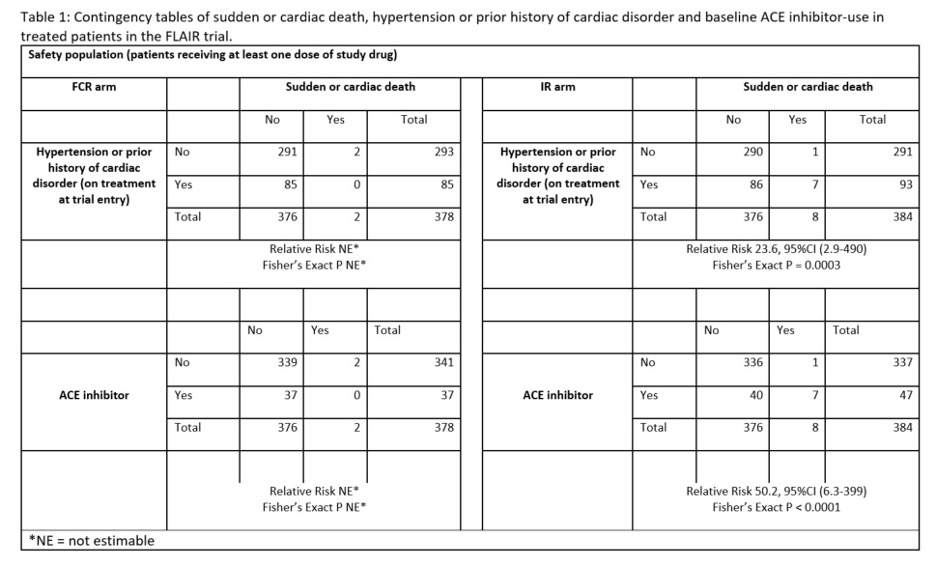
- The relative association of sudden or cardiac deaths in patients treated with IR and the presence or absence of prior history or treatment of a heart or cardiac condition (AF or MI or angina) or HT was evaluated.
- 384 patients received IR, and 378 received FCR therapy. They were followed for a median of 52.7 months FU with treatment durations of 47.2 and 4.7 months.
- There were ten total cardiac deaths, 9 of which occurred in males. In the Ibr arm, there were 8; in the FCR group, there were only two sudden cardiac deaths. The various types and combinations of HT medications were reviewed and stratified according to the number of sudden cardiac deaths in each treatment arm.
- The HT medications included ACE inhibitors (ACEi), beta-blockers, Angiotensin II receptor blockers (ARB’s), calcium channel blockers, diuretics, and alpha-blockers.
- In summary, 47 patients in the IR group were taking ACEi, seven of which died (15%). Two of these had changed to an ABR from ACEi, due to cough, at a point prior to death. Only 1/336 (0.3%) patients not taking an ACEi had a sudden or cardiac death. Further, none of the 46 patients in the IR arm receiving a cardiac medication that was not an ACEi had a sudden cardiac death.
- In the IR group, 3/11 (27%) taking an ACEi plus a beta-blocker vs 4/32 (12.5%) taking only an ACEi at study entry had a sudden or cardiac death.
- In the FCR arm, none of the two patients with sudden or cardiac had a pre-existing cardiac condition or HTN and were not taking an ACEi.
Conclusions:
Patients with sudden or cardiac deaths on IR in FLAIR were predominantly male, had a prior history of cardiac disease or HT, and 7/8 were taking an ACEi, or ACEi plus a beta-blocker. Of those in the IR group not treated with an ACEi, only 1/336 had sudden or cardiac death. Thus, sudden or cardiac death in patients treated with IR is unlikely in the absence of pre-existing cardiac disease or HTN, especially if this was treated with an ACEi alone or in combination with a beta-blocker. If this is confirmed in other trials, this is big news and could offer a simple way to lower the risk of sudden death in CLL / SLL patients on ibrutinib.
Please enjoy this interview with Dr. Talha Munir.
Please enjoy the ASH abstract here.
SMART PATIENTS GET SMART CARE™. Be sure to ask your doctor about the risk factors when considering treatment with Ibrutinib.
Stay Strong. We are all in this together.
Michael Green, MD and CLL patient

















