As the world has seemingly moved on from COVID-19 and appears to be disinterested in hearing about anything related to rates of infection/community spread, hospitalizations, or how many succumb daily to COVID-19, we have noticed that the volume of media coverage and reliable up-to-date information is not making the daily headlines nearly as much as it used to.
However, we are still very much in a pandemic and those who are immunocompromised, including all of those with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL / SLL) regardless of their treatment status, must remain diligent. Without up-to-date information, it is difficult to perform personalized COVID-19 risk assessments to determine what level of risk you are willing to accept for various social situations.
Therefore, CLL Society has decided to try something new throughout the upcoming winter months by providing a weekly updated COVID-19 Summary Report. The goal is to keep our community informed of the latest information available as we approach what Dr. Brian Koffman has referred to as a potential “winter of discontent” for those with CLL / SLL.
Here is our first weekly report (there is a lot of new information this week, so this particular update will be a bit lengthy).
Weekly COVID-19 Statistics
According to the CDC’s map of community level spread, 21.49% of the population lives in an area with medium to high transmission rates. While we are seeing fewer areas with an incredibly high level of spread compared to what was seen over the summer, a multi-layered approach to protection remain necessary to reduce the chance of infection.
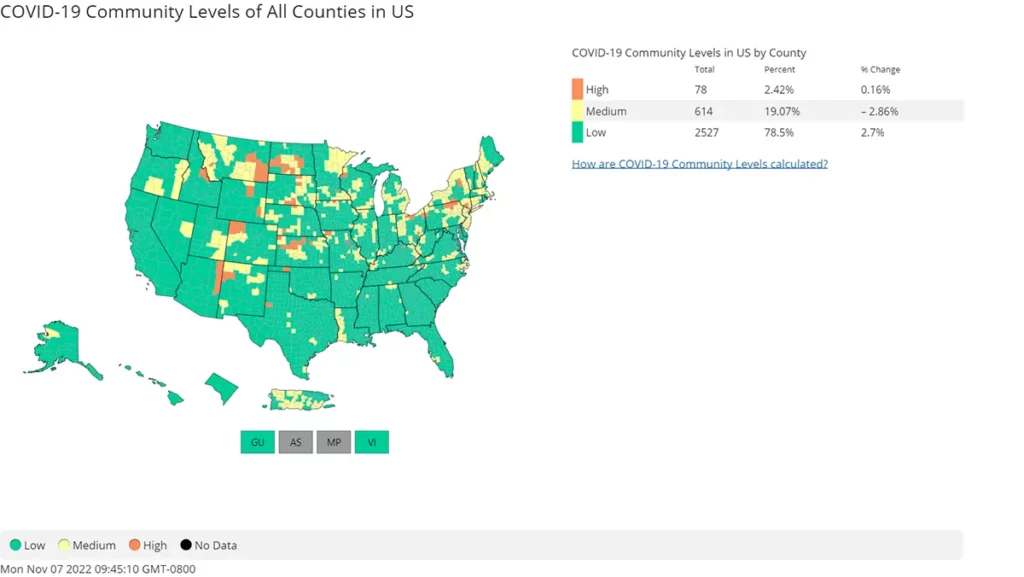
The rate of Hospitalization is mostly flat compared to last week, with some slight increases in the East and Midwest. This is encouraging.
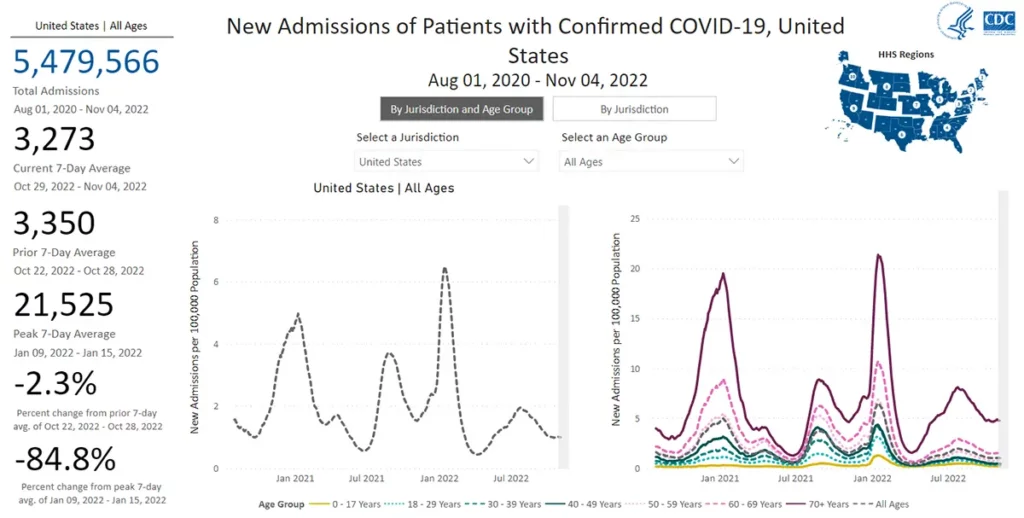
The number of deaths from COVID-19 remains at a plateau of approximately 2,500 on average per week as of November 2nd, 2022.
Current & Emerging Variants of Concern (VOC)
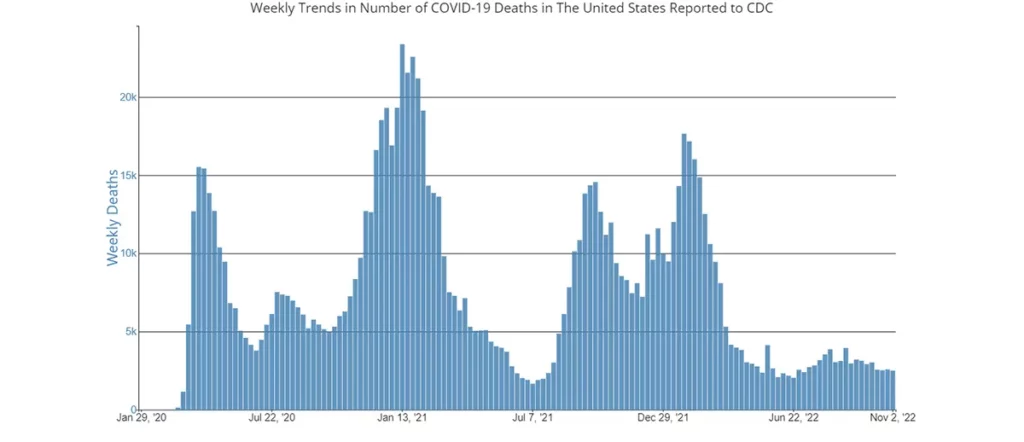
Perhaps the most important news for the CLL / SLL community this week is that on November 4th, the CDC updated its COVID-19 Variant Proportion Data Tracker. BA.5 has been the dominant variant for months now, and was expected to have a slow incremental decline over the coming weeks to months, just as we have often seen before when new variants have appeared in the US.
However, there has been a stunning increase in the number of new variants that suddenly appeared last week, and the rate at which these new variants are out-competing BA.5 is surprising. Here is a graph of the new breakdown of variants.
Many were already aware of this news article and a few others that recently mentioned the only FDA-authorized monoclonal antibodies still left standing, which are Evusheld as a preventative and Bebtelovimab as a treatment. The article touches upon how they could both be deemed ineffective if certain variants of concern emerging in Europe and Asia began to pick up speed in the US. It was thought that there would be a bit more time before their emergence occurred and we would have a little bit more notice to prepare. Unfortunately, they appeared sooner than expected and have grown at a very fast rate. Here is a summary of what we currently know about these newly emerging variants of concern and how they hold up against Evusheld and Bebtelovimab:
It is important to note that both Evusheld and Bebtelovimab are STILL EFFECTIVE against the currently dominant variant of concern, BA.5, which still makes up 39.2% of cases in the US. However, the average number of cases attributed to BA.5 dropped by nearly 11% in just ONE week, which is astonishing.
The preliminary data indicate that Bebtelovimab will no longer be effective against the following variants:
- BQ.1
- BQ.1.1
These two variants together made up over 35% of cases nationwide as of November 4th.
The preliminary data indicate that Evusheld will no longer be effective against the following variants:
- BA.4.6
- BA.2.75.2
- BQ.1
- BQ.1.1
- BF.7
- XBB
These six variants together made up over 55% of cases nationwide as of November 4th.
While the nationwide average of BA.5 is now only 39.2%, there are significant regional differences in the percentage/prevalence of all the variants of concern as seen on the map below. So, it is important to keep an eye on the percentage of variants in your region by watching the breakdown that is updated weekly on the CDC’s variant tracker.
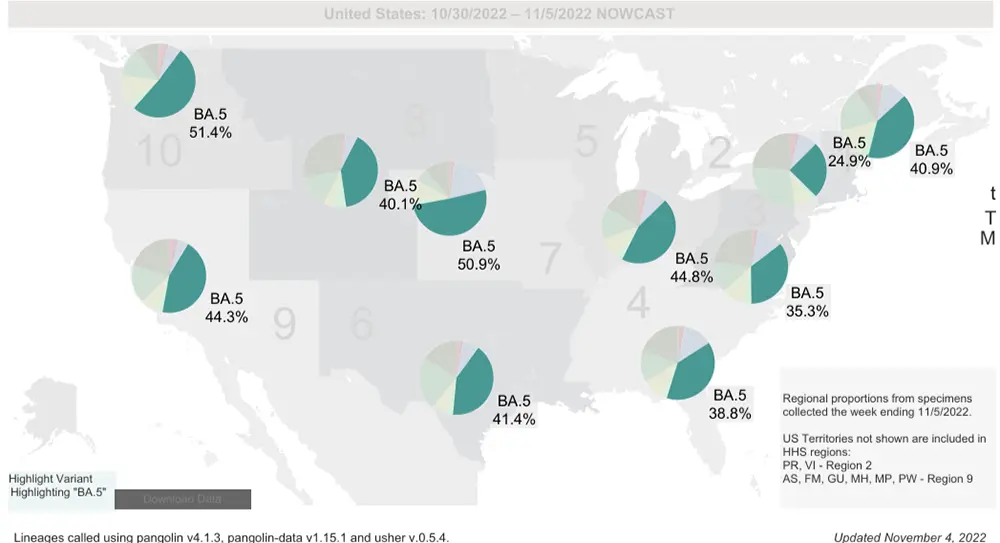
Overall, the most concerning new variant in the US that has emerged is BQ.1.1 and its descendants (such as BQ.1.1.10), since they are the ones showing marked growth advantage right now. Another one to keep an eye on is XBB which is known to have an extreme level of immune evasiveness to natural immunity developed by previous COVID-19 infection and possibly vaccination to some degree (yet to be confirmed).
Some good news is that we can look at what is happening in France, where BQ.1.1 has become dominant and is currently accounting for >50% of new cases. There is no sign yet that it is having a clinical impact resulting in increased hospitalizations. In fact, hospitalizations there are trending down.
COVID-19 in the News This Week
An exciting pre-print was released from the Veterans Administration Health System showing that the use of Paxlovid as a treatment for COVID-19 also reduces the risk of developing Long-COVID by 25%. The study looked at over 9,000 patients treated with Paxlovid within five days of symptom onset. The results showed that the use of Paxlovid not only reduced the toll of Long-COVID, but also the complications that can result from infection, and showed an extended benefit of survival and avoidance of hospitalizations up to one-year post-infection. Before this report, the only way we have known to reduce Long-COVID was to avoid infection, and there was some reduction afforded by prior vaccination and boosters. So, this is some very exciting news!
There was also some great news released from Pfizer/BioNtech reporting that there was an approximate 4-fold increase in the levels of neutralizing antibody directed towards BA.5 compared with the original monovalent Pfizer booster. This is a substantial improvement.
In Summary
As Dr. Koffman predicted, the coming months very well might be our community’s “winter of discontent,” due to the coronavirus doing its very best to continually outsmart us by mutating to the point that it evades previous natural immunity acquired by infection in previous months, evades protection provided by Evusheld, and has figured out how to make the only remaining monoclonal antibody treatment for COVID-19 infection (Bebtelovimab) ineffective.
While Evusheld was never meant to completely prevent COVID-19 infection, it has done an excellent job of preventing individuals from developing severe disease which can lead to hospitalization and death. Without that added layer of protection, CLL Society feels that it is time to prepare those in our community for what might come as a result. We want to stress that this is not a reason to panic or fear, but instead to pause and prepare for what might be coming in the next few weeks.
Please remember that at this time CLL Society still believes that receiving Evusheld and Bebtelovimab is worth obtaining for those who are eligible until the FDA and CDC determine that they are no longer effective!
It is also important to remember that even if Evusheld and Bebtelovimab are no longer tools in our COVID-19 arsenal, we are not being left with zero ways to protect ourselves against infection and severe disease. We still have the powerful oral antiviral Paxlovid and the outpatient option of receiving IV Remdesivir.
Also, we have the new bivalent vaccine, which is still free to the public at this time. However, uptake in the US has been very low with less than 20% of those eligible in the US having received it. If you have not yet obtained your bivalent booster, now is the time to talk with your healthcare provider to discuss receiving it. The bivalent booster may increase antibody levels and stimulate other important parts of the immune system including T cells that aren’t necessarily measurable with standard laboratory tests, even if you haven’t had a previously robust antibody response to other COVID-19 vaccine doses.
In the meantime, we encourage everyone to focus on reducing transmission once again until additional effective monoclonal antibody preventatives and treatments can be developed and authorized. Several are in the pipeline and being studied in clinical trials. CLL Society is communicating with several companies who are working hard to develop new options for us. Stay tuned for more news on this subject, hopefully coming in early 2023.
Thankfully, the virus cannot mutate around a well-fitted N95 mask, so please continue to mask up! Additionally, education and knowledge are both powerful tools, so please do your best to stay well-informed.
Also, make sure your COVID-19 Action Plan is kept up-to-date, have the suggested supplies listed within the plan on hand, practice good hand hygiene, have good ventilation whenever you are around others (especially those who are unmasked), wear an N95 or KN95 as much as possible when outside of your home, and practice social distancing as much as you possibly can.
Together we will get through this, just like we have done for nearly three years now! More information is sure to come, so please stay tuned.
Keep learning and stay well.
Robyn Brumble, MSN, RN
Director of Scientific Affairs & Research
CLL Society



