The update for this week will be abbreviated due to CLL Society staff attending ASH. Numbers have sharply increased this past week and the dominant variants that are not sensitive to Evusheld continue to dominant the variant landscape.
Weekly COVID-19 Statistics
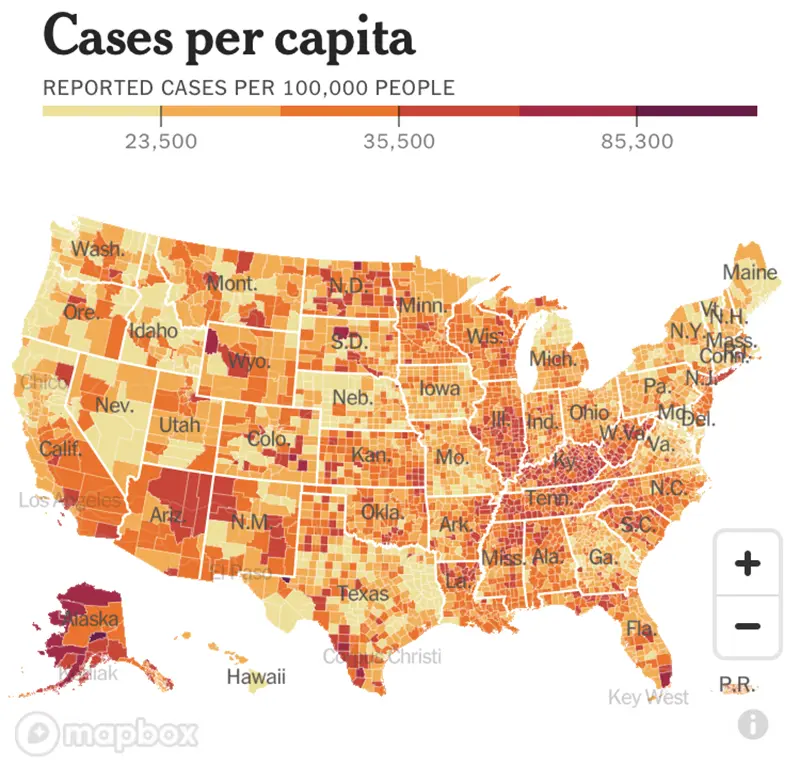
Here is the CDC’s map of community level of spread, and here is the link to the New York Times cases per capita map as shown below.
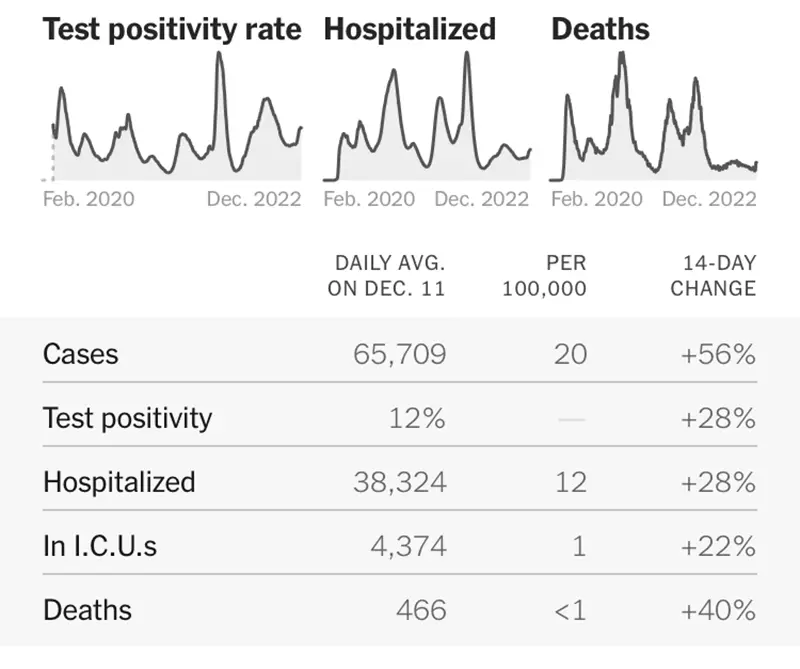
The graphic below is also telling from the New York Times as it shows all data points have increased significantly.
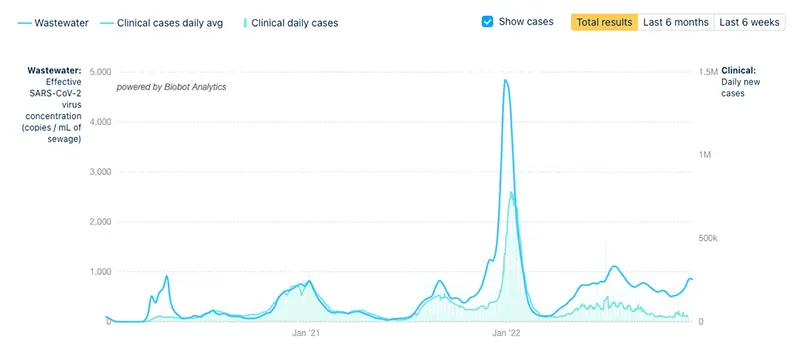
The COVID-19 Wastewater Monitoring Project last updated December 8th also indicates a sharp increase. Note that there is a large gap between the upper dark blue line (wastewater measurements of virus levels) and the lower light blue line (reported case numbers). This verifies that actual case numbers are much higher than what is being reported.
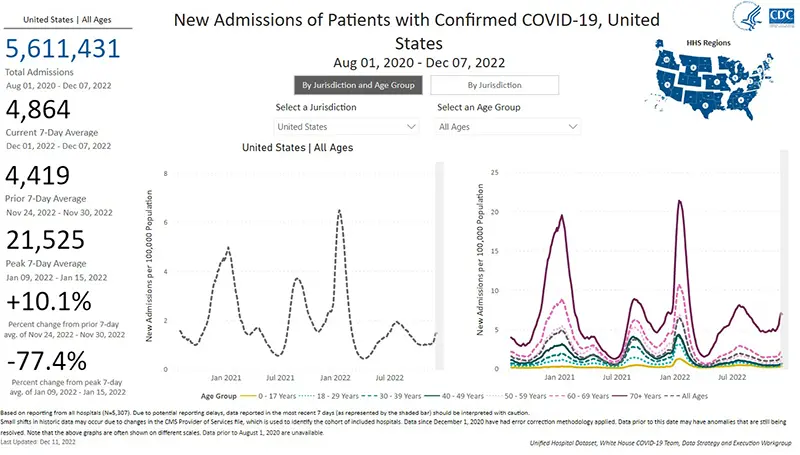
The rate of hospitalizations last updated on December 7th indicate hospitalizations for COVID-19 have gone up when compared to just one week ago. The most concerning data point is that of adults over the age of 70 who are being hospitalized for COVID-19 (as pictured in the graph on the right and are reflected by the dark purple line). This age group continues to have a significantly higher hospitalization rate compared to all other age groups.
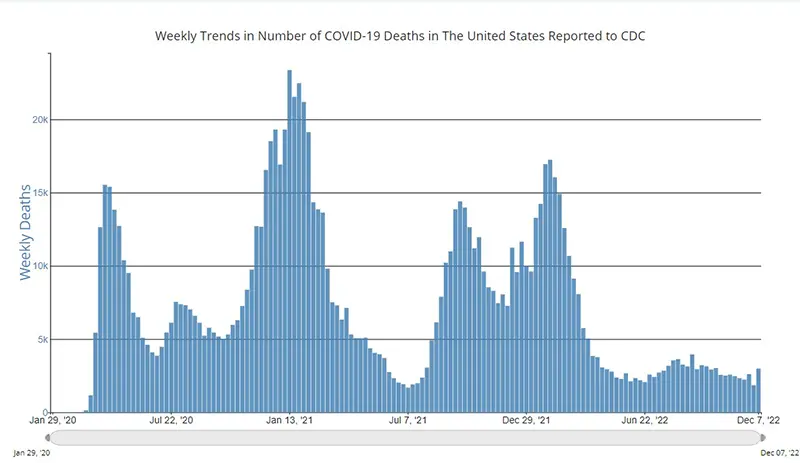
The weekly average number of COVID-19 deaths in the US ending the week of December 7th were 2,981. This is an increase of 1201 deaths from the week prior.
Current & Emerging Variants of Concern (VOC)
BQ.1 and BQ.1.1 combined are now 67% of new cases. Here is the overall breakdown of variants in the US according to the CDC’s Variant Tracker as of December 10th.
And here is the breakdown by regions within the United States as of December 10th. (We continue to highlight how much BA.5 is left by region because it is the main one that Evusheld still works against).
Here is the summary of what we know about the current variants of concern and how they hold up against Evusheld:
Data indicate Evusheld will still provide protection against the following variants (percentages are national averages):
- BA.5 (11.5%)
- BN.1 (4.3%)
- BA.2 (0.7%)
- BA.2.75 (0.6%)
These four variants together make up ~17.1% of cases on average nationwide as of December 10th.
Data indicate that Evusheld will NOT be effective against the following variants (percentages are national averages):
- BQ.1 (31.1%)
- BQ.1.1 (36.8%)
- BF.7 (5.7%)
- XBB (4.7%)
- BA.4.6 (1.6%)
- BA.5.2.6 (1.7%)
- BA.2.75.2 (0.4%)
- BF.11 (0.8%)
These seven variants together made up ~82.2% of cases on average nationwide as of December 10th.
We are hearing multiple reports from around the country that providers and institution are no longer offering Evusheld due to its decreased ability to neutralize the dominant variants. Please discuss any questions you might have surrounding Evusheld with your healthcare provider.
In Summary
- Having Evusheld as a tool to provide those with CLL / SLL an additional layer of protection is no longer something that our community can depend on at this time. CLL Society is in continual contact with our industry partners and we have been told that several companies are working on developing new formularies of COVID-19 monoclonal antibodies that could ideally be used as pre-exposure prophylaxis (like Evusheld was) as well as for treatment, which is very encouraging. But these new COVID-19 monoclonal antibodies are not even in early clinical trials yet. So the earliest we might expect them to be available to the public could be fall/winter 2023 at best.
- If you have known exposure to COVID-19 or are experiencing any symptoms at all, please get tested early and call your healthcare provider. Preferably obtain a PCR test which can more reliably detect presence of the virus), so that you can have adequate time to receive treatments that can prevent the development of severe disease. Paxlovid must be started orally within five days of symptom onsetand Remdesivir must be started intravenously within seven days of symptom onset.
- Please obtain your bivalent booster if you are eligible and have not already done so. Now is the time! Even if you have not historically had a robust spike protein antibody response, we now know that the vaccine stimulates other important parts of the immune system such as memory T-cells that are also important for fighting off COVID-19 infection.
- Wear minimally a well-fitted KN95 mask or most preferably an N95 mask while around others who live outside of your household. Surgical masks and cloth masks will not protect you if someone around you is infectious. You can obtain quality N95 masks free of charge from many local pharmacies across the country.
- Practice social distancing and avoid indoor gatherings or crowded situations as much as possible.
- Please revisit your COVID-19 Action Plan.
- Practice good hand washing often and use hand sanitizer.
- Ensure there is good air-flow and ventilation whenever you are around others by opening windows or doors as weather allows, and/or using a HEPA air purifier if you have one available.
Keep learning, and please stay well.
Robyn Brumble, MSN, RN
Director of Scientific Affairs & Research
CLL Society

















