As expected, after much of the US gathered indoors for the Thanksgiving holiday weekend, COVID-19 numbers are once again headed in the wrong direction after an autumn lull. Hospitalizations have jumped by 25% in recent days, and we are already seeing increases in test positivity, wastewater surveillance virus levels, and even cases-which we have mentioned in prior updates are grossly under-reported due to the wide availability of at home rapid antigen testing.
Weekly COVID-19 Statistics
This week, we are going to keep the link for the CDC’s map of community level of spread, but for the graphic we are switching to the New York Times, which more accurately reflects case counts per capita. This is because the CDC’s map does not reflect actual case counts, but instead weights the data based on hospitalizations. We feel the New York Times map will give our immunocompromised community a better way to gauge their level of risk.
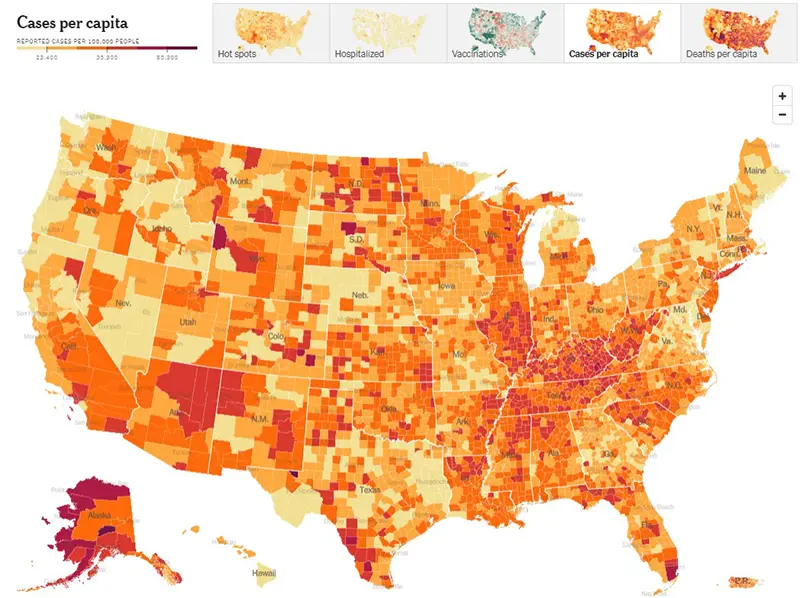
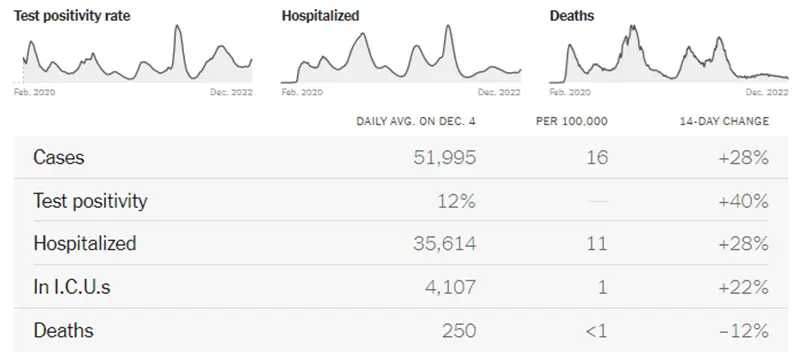
The graphic below (also from the New York Times) is very telling. All data points have increased significantly except for deaths, which is always a lagging indicator behind cases and hospitalizations by about two weeks.
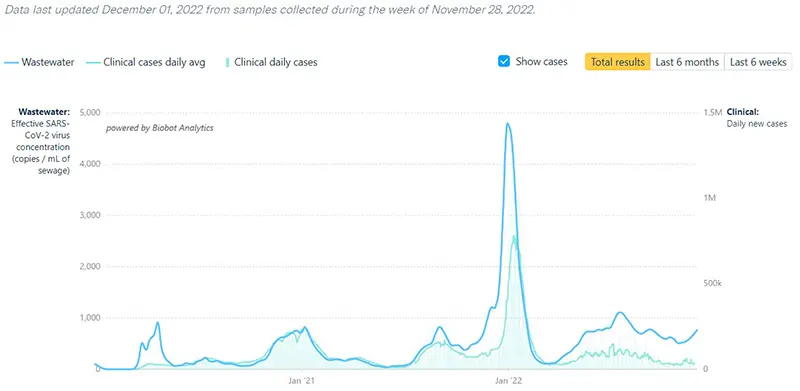
The COVID-19 Wastewater Monitoring Project data that was last updated December 1st also indicates a sharp increase. Again, note that there is a large gap between the upper dark blue line (wastewater measurements of virus levels) and the lower light blue line (actual reported case numbers). This data point verifies that actual case numbers are much higher than what is being reported.
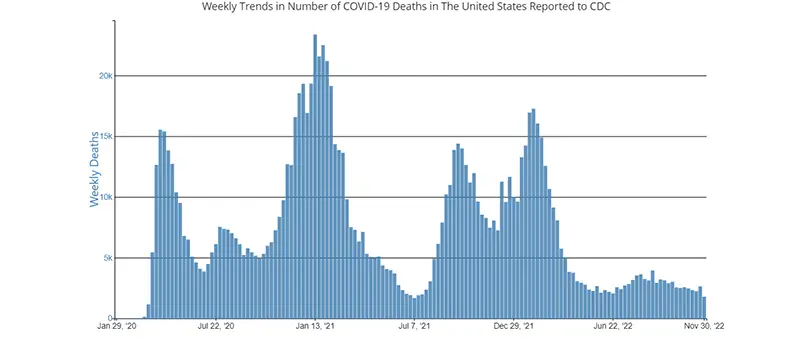
The rate of hospitalizations last updated on December 2nd indicate hospital admissions for COVID-19 have gone up a whopping 27% compared to just one week ago. Note that when hospitalizations are broken down by age groups (as pictured below in the graph on the right), those over the age of 70 (reflected by the dark purple line) continue to have a significantly higher hospitalization rate compared to all other age groups. Those over the age of 70 also have the sharpest rise in hospitalizations this week.

Less than one in three seniors have received the bivalent COVID-19 booster since it became available three months ago, and less than 15% of Americans have received it as of the time this article was written. Study after study has indicated having had a COVID-19 booster within the past 4-6 months decreases a person’s chance of being hospitalized by up to 80%. The poor uptake of the bivalent vaccine by Americans will undoubtedly be a large factor that plays into the upcoming winter surge.
The weekly average number of COVID-19 deaths in the US ending the week of November 30th were 1,780. This is a decrease of 864 deaths from the week prior. As mentioned above, historically throughout the pandemic we have witnessed that it takes approximately two weeks after case numbers and hospitalizations rise to begin seeing an increase in COVID-19 deaths.
Current & Emerging Variants of Concern (VOC)
BQ.1 and BQ.1.1 combined now make up to 63% of new cases. The BA.5 wave has faded away while the new (somewhat concerning) XBB variant is gaining ground slowly. XBB is thought by some scientists to possibly evade natural immunity (and to some degree vaccinations), but there are no confirmatory data yet. BQ.1, BQ.1.1, and XBB are all three resistant to Evusheld. Here is the overall breakdown of variants in the US according to the CDC’s Variant Tracker as of December 3rd.
And here is the breakdown by region. (We continue to highlight how much BA.5 is left by region because it is the one that Evusheld still works against. For example, Evusheld is only going to help against ~6.9% of COVID-19 cases in the New York State region).
Here is the summary of what we know about the current variants of concern and how they hold up against Evusheld (we are removing the analyses of Bebtelovimab since it is no longer available in the US as of this week):
Data indicate Evusheld will still provide protection against the following variants (percentages are national averages):
- BA.5 (13.8%)
- BN.1 (4.6%-based on preliminary data)
- BA.2 (0.8%)
- BA,2.75 (0.7%)
These four variants together make up 19.9% of cases on average nationwide as of December 2nd.
Data indicate that Evusheld will NOT be effective against the following variants (percentages are national averages):
- BQ.1 (30.9%)
- BQ.1.1 (31.9%)
- BF.7 (6.3%)
- XBB (5.5%)
- BA.4.6 (2.3%)
- BA.5.2.6 (1.8%)
- BA.2.75.2 (0.5%)
These seven variants together made up 79.2% of cases on average nationwide as of December 2nd.
CLL Society is still waiting to see if a formal announcement will be forthcoming from the FDA on Evusheld. Until that time, please discuss any questions you might have surrounding Evusheld with your healthcare provider. We are hearing multiple reports from around the country that providers and institutions are no longer providing the six-month booster doses of Evusheld at this time.
COVID-19 in the News
A large study published in JAMA indicated the occurrence of myocarditis (inflammation of the heart muscle) and pericarditis (inflammation of the outer lining of the heart) after COVID-19 vaccination is extremely low, most patients make a full recovery, and zero deaths were observed. The majority of cases occurred in male teens and young adults after the second dose of the primary series of mRNA vaccines. The authors also noted that the risk of developing heart inflammation after COVID-19 infection is significantly higher than after vaccination.
The FDA pulled Bebtelovimab as COVID-19 antibody treatment because it is no longer effective. We wrote an article about this that can be read in full here.
In Summary
- With Bebtelovimab no longer being available for treatment and with the decreased effectiveness of Evusheld, if you have known exposure to COVID-19 or are experiencing any symptoms at all, please get tested early and call your healthcare provider. Preferably obtain a PCR test (which can more reliably detect presence of the virus even before symptoms develop), so that you can have adequate time to receive treatments that can prevent the development of severe disease. Paxlovid must be started orally within five days of symptom onset, and Remdesivir must be started intravenously within seven days of symptom onset.
- Please obtain your bivalent booster if you are eligible. Now is the time! Even if you have not historically had a robust spike protein antibody response, we now know that the vaccine stimulates other important parts of the immune system (such as memory T-cells) that are also very important in fighting off COVID-19 infection.
- Minimally wear a well-fitted KN95 mask, or most preferably an N95 mask, while around others who live outside of your household. Surgical masks and cloth masks do not effectively protect you from the virus if someone around you is infectious. You can obtain quality N95 masks free of charge from many local pharmacies across the country.
- Practice social distancing and avoid indoor gatherings or crowded situations as much as possible.
- Please revisit your COVID-19 Action Plan.
- Practice good hand washing often and use hand sanitizer.
- Ensure there is good air-flow and ventilation whenever you are around others indoors by opening windows and doors as weather allows, and/or by using a HEPA air purifier if you have one available.
Keep learning, and please stay well.
Robyn Brumble, MSN, RN
Director of Scientific Affairs & Research
CLL Society

















