Immunocompromised and worried about COVID-19? If you recently had your COVID-19 vaccine booster, you may be eligible for free antibody testing to understand your antibody levels, as part of a research registry for potential participation in a COVID-19 prevention study of investigational monoclonal antibody therapies. Click here to learn more.
COVID-19 Statistics
Here is this week’s CDC map of community level of spread (this particular link is weighted based on hospitalizations and not based upon the actual level of community spread). And here is the link to the New York Times actual cases per capita map as pictured below. The New York Times data was last updated February 20th.
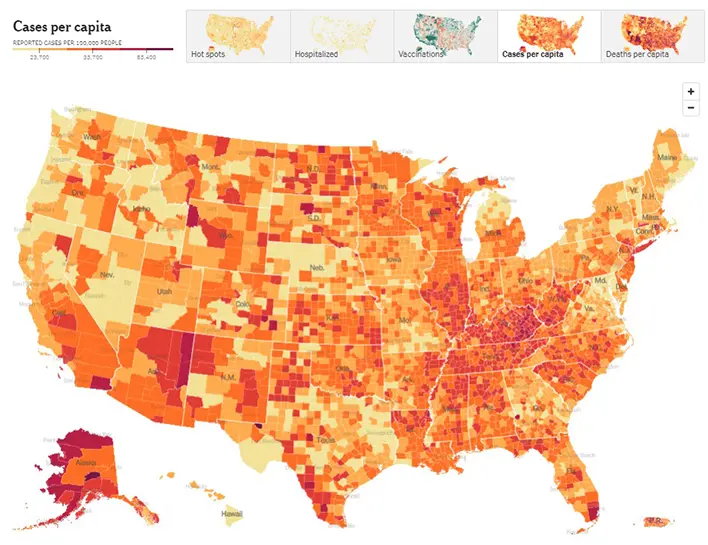
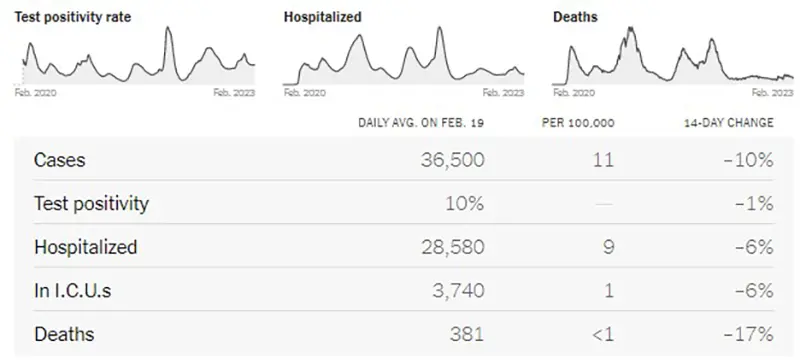
Here is the summary graphic from the New York Times as of February 20th. We continue to see a downward trend in all 14-day change metrics, although test positivity has plateaued.
Here is a graphic from the Wastewater Monitoring Project last updated on February 16th. This graphic does not reflect necessarily a decrease in the amount of SARS-CoV-2 virus being detected in wastewater, but instead, more of a plateau. Sadly, the current plateau appears that it might be set at the highest plateau level seen since the beginning of the pandemic.
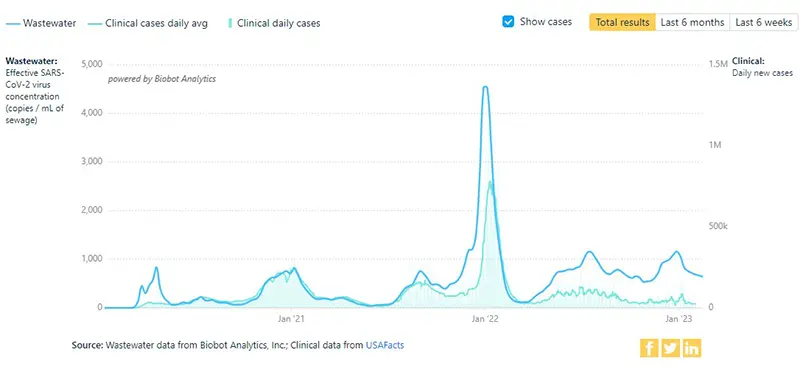
The additional graphic below from the Wastewater Project shows SARS-CoV-2 viral concentrations by region. All regions are also beginning to reflect a plateau rather than a downward trend, with the highest concentration of SARS-CoV-2 virus concentration amounts remaining in the Northeast region of the country.

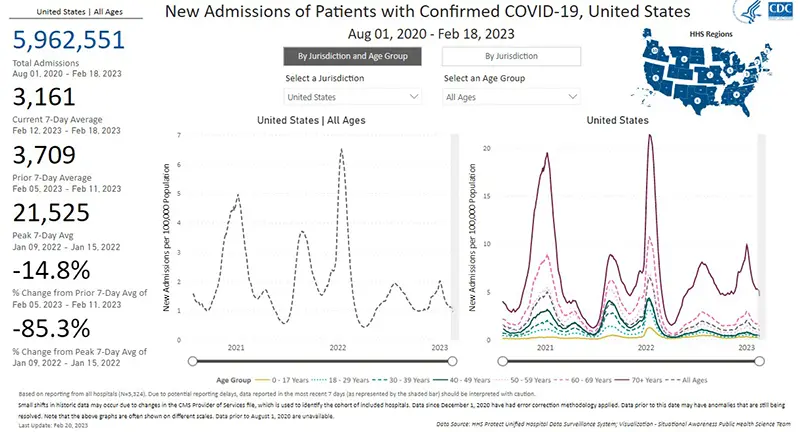
Hospitalizations due to COVID-19, last updated February 18th, overall appear to be plateauing as well (gray dotted line graphic on the left). However, although hospitalizations by age group have all gone down compared to December, there continues to be a disproportionately high number of hospitalizations for those over the age of 70 (purple solid line on the right) compared to all of those in younger age groups.
The weekly average number of COVID-19 deaths according to the CDC in the US ending February 15th was 2,838. This is a slight decrease from the week prior, but we still have not returned to the lower baseline as seen in November.

Current & Emerging Variants of Concern (VOC)
As of February 18th, XBB.1.5 (also called the Kraken variant) makes up 80.2% of total cases in the US, surpassing last week’s total of 74.7%, with no other variant showing gains. The good news is that there has not been an uptick in hospitalizations or deaths as a result of XBB.1.5 being dominant now across the entire US.
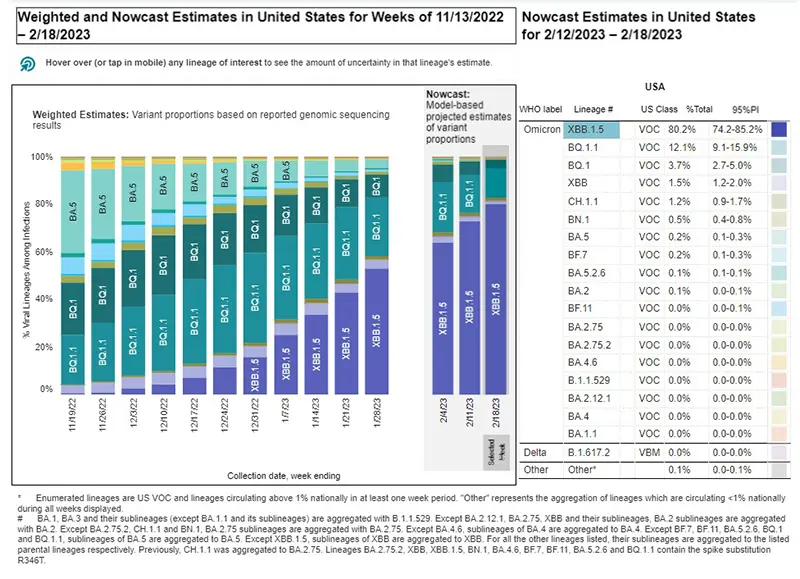
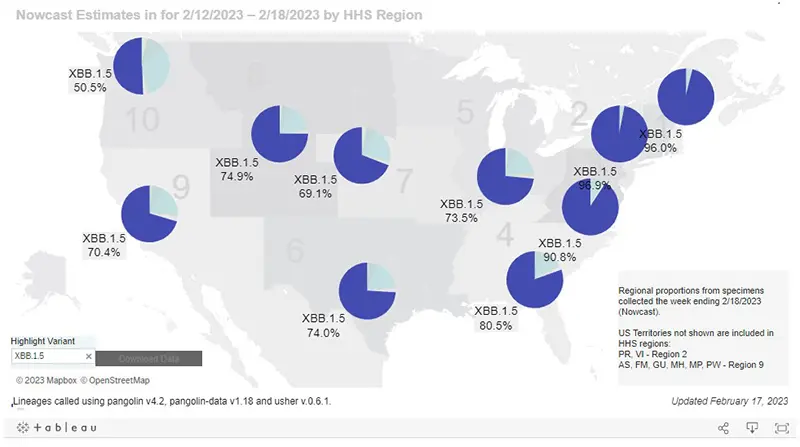
XBB.1.5 has spread across the country incredibly fast due to it being highly infectious. It now makes up more than half of the cases in all regions of the US (and more than 75% in regions 1-4).
COVID-19 in the News
- Moderna announced in a statement that people will not need to pay out-of-pocket for it’s COVID-19 vaccine, regardless of their insurance status, once the public health emergency declaration expires in May. Unfortunately, free Moderna vaccines will be provided to the underinsured through its patient assistance program, which will require an application process.
- The CDC published a Morbidity and Mortality Weekly Report (MMWR) on February 10th indicating those who received the bivalent booster vaccine were 14 times less likely to die from COVID-19 when compared to those who were unvaccinated. Additionally, the report showed those who received the bivalent booster were three times less likely to die from COVID-19 compared to those who only received the monovalent vaccine series.
- The FDA updated the Emergency Use Authorization Fact Sheet for Paxlovid. It is no longer a requirement for an individual to have already tested positive for COVID-19 for their healthcare provider to prescribe them Paxlovid. This is because the FDA recognizes that in some instances, people with a recent known exposure (such as to someone in their household who has already tested positive for COVID-19) may not test positive with home antigen tests for many days into their infection, despite having symptoms. In such instances, the authorized prescriber may determine that treatment with Paxlovid for COVID-19 is appropriate if the patient reports mild-to-moderate symptoms of COVID-19 and is at high-risk for progression to severe COVID-19. This is an incredibly positive change, as Paxlovid must be started within the first five days of symptom onset!
Keep learning, and please stay well.
Robyn Brumble, MSN, RN
Director of Scientific Affairs & Research
CLL Society



