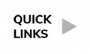Bottom Line:
This meta-analysis or combination of 61 trials demonstrated that atrial fibrillation, hypertension, and diarrhea were more common with ibrutinib, and some bleeding and infection events were more common with acalabrutinib and zanubrutinib.
Who Performed the Research and Where Was it Presented:
A consortium of doctors led by Dr. Jacqueline Wang of NYU Langone Health presented the abstract at ASH 2022.
Background:
We are aware of how Bruton’s tyrosine kinase inhibitors (BTKi’s) have revolutionized the therapeutic landscape of B-cell malignancies, including chronic lymphocytic leukemia/small lymphocytic leukemia (CLL / SLL), mantle cell lymphoma (MCL), and Waldenstrom’s macroglobulinemia (WM). Ibrutinib was the first FDA-approved BTKi. Unfortunately, its off-target effects have resulted in several adverse events (AEs) of concern, such as atrial fibrillation (an irregular heart rhythm), hypertension, hemorrhage, and diarrhea. Acalabrutinib and zanubrutinib are second-generation, more selective BTKIs developed to minimize off-target effects. While multiple trials have been conducted for these BTKi’s in B-cell malignancies, direct comparison of toxicity profiles between BTKi’s has been limited to just three trials. The purpose of this study was to augment our understanding of these side effects reported with the three approved BTKIs by compiling together many clinical trials.
Methods:
PubMed and major hematology/oncology conference abstracts such as ASH or EHA, or ASCO were searched (last updated query on July 11, 2022) for trials of ibrutinib, acalabrutinib, and zanubrutinib in B-cell malignancies. In addition, adverse events (AEs) of clinical interest or AEs reported by ≥10% of the included trials were analyzed.
Results:
- A total of 61 trials were included involving 6458 patients and 68 treatment arms: ibrutinib (n=30; 44%), ibrutinib plus CD20-mAb (n=14; 21%), acalabrutinib (n=11; 16%), acalabrutinib plus CD20-monoclonal antibodies (mAb) such as rituximab (n=2; 3%), zanubrutinib (n=10; 15%), and zanubrutinib plus CD20-mAb (n=1; 1%).
- More than half of the trials were in CLL / SLL (n=32), the rest in MCL (n=10) or WM (n=9);
- 38 trials were relapsed/refractory setting and 14 in frontline.
- Only three trials involved a randomized comparison between different BTKis (ASPEN, ELEVATE-RR, and ALPINE).
- A total of 65 different AEs were analyzed.
- The most common all-grade AEs across all drugs and trials were infection (62.2%), hemorrhage (41.5%), diarrhea (34.3%), fatigue (22.8%), neutropenia or low neutrophil count (21.6%), and upper respiratory infection (URI) (21.0%).
- The most common grade ≥3 or more serious AEs included neutropenia or low neutrophil count (14.9%) and infection (12.3%).
- All grade/grade ≥3 atrial fibrillation incidences were 6.0%/2.5%, respectively, and for hypertension were 12.5%/7.8%, respectively.
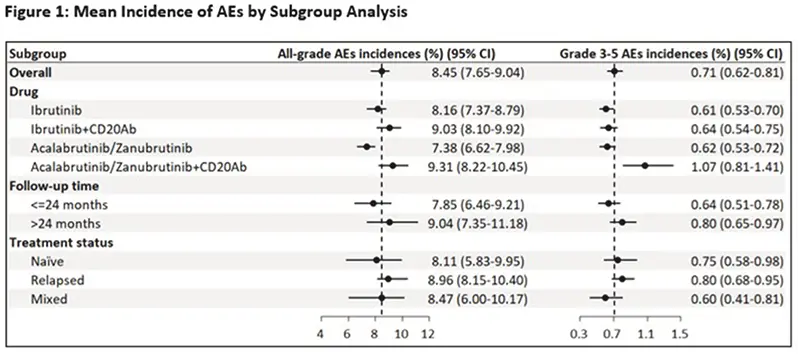
- Comparisons between AEs in trials resulting from ibrutinib and AEs in either acalabrutinib or zanubrutinib were made.
- AEs of any grade that occurred more frequently with ibrutinib included atrial fibrillation (acalabrutinib/zanubrutinib vs. ibrutinib odds ratio or OR 0.30), hypertension (OR 0.47), anemia (OR 0.67), low platelets or thrombocytopenia (OR 0.57), nausea (OR 0.67), vomiting (OR 0.77), and diarrhea (OR 0.49).
- Grade ≥3 or more severe AEs that occurred more frequently with ibrutinib included atrial fibrillation (OR 0.29), hypertension (OR 0.37), diarrhea (OR 0.52), and rash (OR 0.30).
- All grade AEs that occurred more frequently with acalabrutinib/zanubrutinib included contusions (OR 1.50), hematuria (blood in the urine) (OR 1.96), leukopenia (low white blood cell count (OR 2.93), second primary malignancies (OR 1.53), upper respiratory infections such as the common cold (OR 1.29), and headaches (OR 2.03). The risk of headaches when starting acalabrutinib is well known.
- Grade ≥3 AEs that occurred more frequently with acalabrutinib/zanubrutinib included leukopenia (low white blood cell counts) (OR 2.08), upper respiratory infections (OR 1.74), and pneumonia (OR 1.35).
Conclusions:
This is the largest meta-analysis of AEs reported with covalent BTKIs. The results help us better understand the side effects of BTKIs in CLL and other related B-cell lymphomas and cancers. Atrial fibrillation, hypertension, and diarrhea were more common with ibrutinib, similar to what was seen in the head-to-head randomized trials. Additionally, bleeding and infection were more common with acalabrutinib/zanubrutinib. This needs further study to determine whether it is a valid finding.
Links and Resources:
Watch my review of this abstract:
The actual trial abstract is: Comparison of Treatment-Emergent Adverse Events of Covalent BTK Inhibitors in Clinical Trials in B-Cell Malignancies: A Systematic Review and Meta-Analysis.
Stay strong. We are all in this together.
Brian
Brian Koffman MDCM (retired) MS Ed (he, him, his)
Co-Founder, Executive VP, and Chief Medical Officer
CLL Society, Inc.