
By Chris Pleyer, MD
Patients with chronic lymphocytic leukemia (CLL) have an increased risk of infection due to a weakened immune system1. At least one third of CLL patients are expected to have one or more major infections during the course of their disease2,3.
Vaccinations are a safe and effective way to boost immunity and prevent infections. Although we know a lot about vaccine responses in the general population, patients with immunocompromising conditions, including CLL, are usually excluded from large vaccine trials. We, therefore, know little about how well CLL patients respond to vaccines. It is generally thought that vaccinations are less effective with immunocompromised individuals as compared to healthy individuals. Previous studies found that 15-63% of CLL patients responded to seasonal influenza (flu) vaccinations4,5 and 20-70% of CLL patients respond to pneumonia vaccinations.6-8 Chemotherapy can further reduce the chance of responding to vaccines9. In addition, it is unclear to what extent CLL patients, treated with Bruton’s Tyrosine Kinase (BTK) inhibitors, (e.g. ibrutinib and acalabrutinib), respond to vaccines. In two small studies, 7-26% of CLL patients treated ibrutinib responded to influenza vaccinations.10,11
Because of the uncertainty surrounding the efficacy of vaccines, some doctors do not recommend vaccines to their CLL patients, whereas others recommend age-appropriate vaccinations. Although there are no formal vaccination guidelines specifically for CLL patients, the Centers for Disease Control Advisory Committee on Immunization Practices provides vaccine recommendations for immunocompromised individuals, which are applicable to CLL patients.
To study how well CLL patients respond to vaccines, we are conducting clinical studies at the NIH in Bethesda, MD. We are using two different vaccines, HEPLISAV-B (hepatitis B vaccine) and SHINGRIX (shingles vaccine) that will allow us to study different types of immune responses. The two vaccines were chosen because they both are safe and in patients who respond, provide important protection against viral infections.
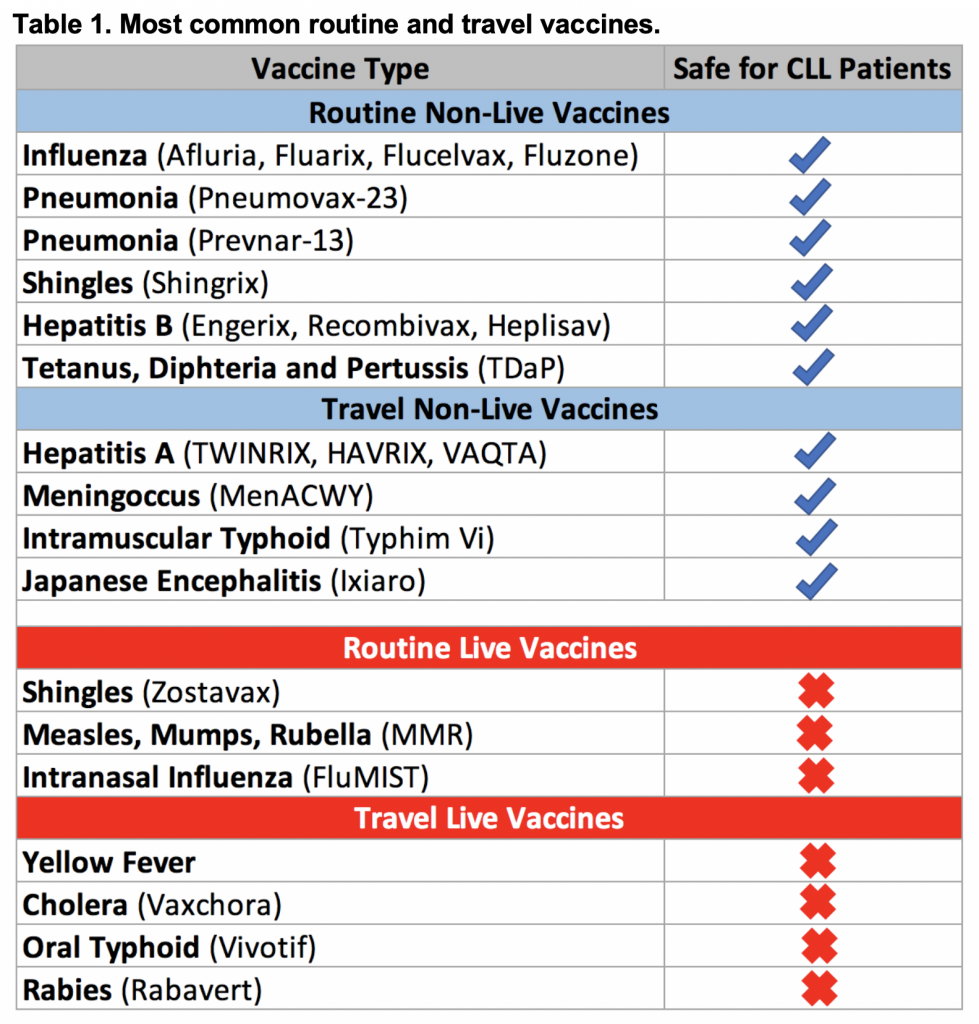
General recommendations for CLL patients include live vaccines that should be avoided due to a risk of disease reactivation following vaccination. Non-live vaccines are considered safe to administer to patients with CLL. The need for travel vaccines is highly individualized and depends on the area patients are traveling to, the length of exposure, as well as planned activities. If CLL patients are planning a trip abroad, the need for specific travel vaccines are best discussed with an infectious disease and/or travel medicine specialist. Table 1 provides a summary of the most common routine and travel vaccinations. More detailed information can be found on the CDC website.
In summary, infections have a major impact on the course of CLL patients, regardless of treatment status. Vaccinations effectively prevent infections in the general population, but we need to learn more about vaccine response in CLL patients, particularly with the use of BTK inhibitors. Until more evidence becomes available, specifically for CLL patients, it is reasonable to follow vaccination guidelines that the CDC provides for immunocompromised individuals.
Influenza Vaccine: Influenza, commonly known as the flu, is an acute respiratory illness that occurs worldwide, mainly during the winter season. Patients who are elderly or who are immunocompromised have an increased risk of dying from influenza. An annual vaccination for influenza is recommended for all adults. Small studies investigating the influenza vaccine in CLL patients have found highly variable vaccine response rates (see above) 4,5. There are several annual influenza vaccine products commercially available (e.g. Afluria, Fluarix Flucelvax, Fluzone). There is also a high-dose influenza vaccine available (Fluzone High-Dose), which is modestly more effective than the standard dose of Fluzone in healthy older adults12. Despite this, the CDC has no preference for any one flu vaccine type. Any age appropriate flu vaccine is acceptable. One of the reasons for having no preference for a specific flu vaccine type is not to delay vaccine administration if a specific product is not immediately available in the clinic. The risk of delaying vaccine administration is thought to outweigh the small potential benefit in vaccine response rates with the high-dose influenza vaccine.
Pneumonia Vaccine: Pneumonia is an acute respiratory infection most commonly caused by viruses, bacteria or fungi. There are two vaccines available to help prevent pneumonia caused by the bacteria Streptococcus pneumoniae. Pneumovax-23 (PPSV) and Prevnar-13 (PCV) protect against different strains of Streptococcus pneumonia. Vaccine response rates in CLL following Prevnar-13 and/or Pneumovax-23 vaccination have also been variable (see above)6-8. Immunocompromised adults, including patients with CLL are recommended to receive both the Prevnar-13 and the Pneumovax-23 vaccine. The Pneumovax-23 can be given as an additional booster dose 5 years after the first vaccination. Practices vary on whether to give repeated booster doses every 5 years. In our practice, we generally recommend patients to receive Pneumovax-23 every 5 years. There is currently no recommendation to receive additional booster vaccinations with Prevnar 13.
Shingles Vaccine: Shingles is painful rash that is caused by reactivation of the varicella zoster virus (VZV). Almost 1 out of 3 people in the United States will develop shingles during their lifetime. Patients who are elderly and/or are immunocompromised have a higher risk of developing shingles. Patients with CLL should not receive the live shingles vaccine (ZOSTAVAX). There is, however, a new, non-live shingles vaccine (SHINGRIX) that can be given to patients with CLL. Two large trials showed that healthy patients who received SHINGRIX achieved 90% protection from developing shingles 13,14. SHINGRIX is recommended for everyone ³50 years old, including patients who received ZOSTAVAX, and patients with a history of shingles. There is currently an ongoing clinical trial investigating the efficacy of the SHINGRIX vaccine in patients with CLL.
Hepatitis B Vaccine: Hepatitis B is a virus that can cause liver damage (cirrhosis), liver failure, and liver cancer. The virus is transmitted through blood and can be acquired through sex with an infected partner, as well as contact with contaminated material including needles, syringes, medical equipment, and blood products. Individuals with frequent interactions with the healthcare system and individuals with chronic medical conditions, are recommended to receive the Hepatitis B vaccination. There is currently an ongoing clinical trial investigating the HEPLISAV-B Hepatitis B vaccine in patients with CLL.
Tetanus, Diphtheria, and Pertussis (Tdap) Vaccine: Tetanus is a nervous system disorder characterized by muscle spasms. Diphtheria is a bacterial infection that can affect the lungs or skin. Pertussis, also known as whooping cough, is an acute respiratory illness. All adults are recommended to receive a booster vaccine every 10 years to assure ongoing protection against tetanus and diphtheria. There are currently no studies studying the Tdap vaccine in CLL.
Acknowledgments
The author is supported by the intramural program of the NHLBI, NIH.
Disclosure of Conflicts of Interest
The author has no disclosures or conflicts of interest to report.
References and suggested further reading
- Morrison VA. Infectious complications of chronic lymphocytic leukaemia: pathogenesis, spectrum of infection, preventive approaches. Best practice & research Clinical haematology 2010; 23(1): 145-53.
- Molica S. Infections in chronic lymphocytic leukemia: risk factors, and impact on survival, and treatment. Leukemia & lymphoma 1994; 13(3-4): 203-14.
- Williams AM, Baran AM, Meacham PJ, et al. Analysis of the risk of infection in patients with chronic lymphocytic leukemia in the era of novel therapies. Leukemia & lymphoma 2018; 59(3): 625-32.
- Gribabis DA, Panayiotidis P, Boussiotis VA, Hannoun C, Pangalis GA. Influenza virus vaccine in B-cell chronic lymphocytic leukaemia patients. Acta Haematol 1994; 91(3): 115-8.
- van der Velden AM, Mulder AH, Hartkamp A, Diepersloot RJ, van Velzen-Blad H, Biesma DH. Influenza virus vaccination and booster in B-cell chronic lymphocytic leukaemia patients. Eur J Intern Med 2001; 12(5): 420-4.
- Lindstrom V, Aittoniemi J, Salmenniemi U, et al. Antibody persistence after pneumococcal conjugate vaccination in patients with chronic lymphocytic leukemia. Human vaccines & immunotherapeutics 2018; 14(6): 1471-4.
- Sinisalo M, Vilpo J, Itala M, Vakevainen M, Taurio J, Aittoniemi J. Antibody response to 7-valent conjugated pneumococcal vaccine in patients with chronic lymphocytic leukaemia. Vaccine 2007; 26(1): 82-7.
- Svensson T, Kattstrom M, Hammarlund Y, et al. Pneumococcal conjugate vaccine triggers a better immune response than pneumococcal polysaccharide vaccine in patients with chronic lymphocytic leukemia A randomized study by the Swedish CLL group. Vaccine 2018; 36(25): 3701-7.
- Safdar A, Rodriguez GH, Rueda AM, et al. Multiple-dose granulocyte-macrophage-colony-stimulating factor plus 23-valent polysaccharide pneumococcal vaccine in patients with chronic lymphocytic leukemia: a prospective, randomized trial of safety and immunogenicity. Cancer 2008; 113(2): 383-7.
- Douglas AP, Trubiano JA, Barr I, Leung VK, Slavin MA, Tam CS. Ibrutinib may impair serological responses to influenza vaccination. Haematologica 2017.
- Sun C, Gao J, Couzens L, et al. Seasonal Influenza Vaccination in Patients With Chronic Lymphocytic Leukemia Treated With Ibrutinib. JAMA Oncol 2016; 2(12): 1656-7.
- DiazGranados CA, Dunning AJ, Kimmel M, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. The New England journal of medicine 2014; 371(7): 635-45.
- Cunningham AL, Lal H, Kovac M, et al. Efficacy of the Herpes Zoster Subunit Vaccine in Adults 70 Years of Age or Older. The New England journal of medicine 2016; 375(11): 1019-32.
- Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. The New England journal of medicine 2015; 372(22): 2087-96.
Christopher Pleyer, MD, is an attending physician at the Hematology Branch, National Heart, Lung, and Blood Institute (NHLBI) and an investigator in the Laboratory of Lymphoid Malignancies, all within the National Institutes of Health (NIH). Dr Pleyer’s current research is focused on i) improving supportive care for CLL patients, ii) assessing immunologic changes in CLL treated with B-cell receptor pathway inhibitors, and iii) assessing clonal dynamics with novel CLL therapies.
Originally published in The CLL Society Tribune Q4 2019.

















