Introduction:
At the 2021 annual meeting of the American Society of Hematology (ASH), Dr. Brian Hill of the Cleveland Clinic interviews Dr. Jacqueline Barrientos, MD, MS from the division of Medical Oncology and Hematology, Northwell Health Cancer Institute Donald & Barbara Zucker School of Medicine at Hofstra/Northwell, Lake Success, NY. They discussed how well community practices follow the National Comprehensive Cancer Network (NCCN) guidelines in testing and treating chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL / SLL) with the poor prognostic markers del(17p)/TP53 mutations, elderly with comorbidities, relapsed or refractory disease (RR) and younger patients without the comorbidities.
Takeaways:
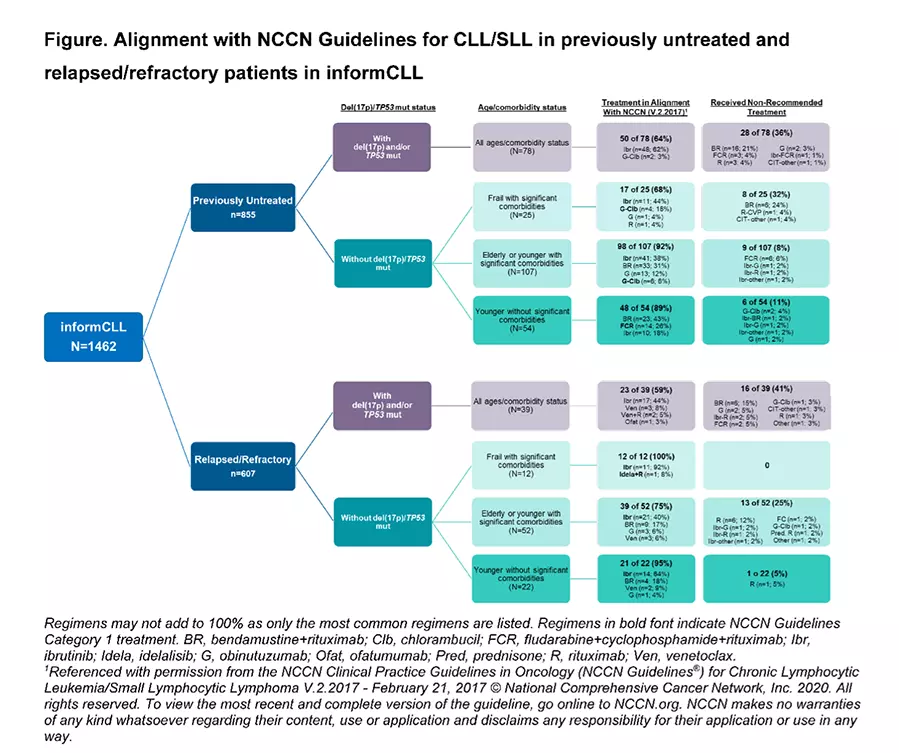
- A total of 1462 CLL / SLL patients over the age of 18 receiving treatment between October 2015 and June 2018 were evaluated for compliance with the 2017 NCCN guidelines. 58% had not been previously treated or were receiving first-line (1 L), while 42% had therapy for relapsed or refractory disease.
- The median age was 71 with a range of 34-95 years.
- Of this group, 93% were treated in community-based practices instead of a CLL specialty clinic or university.
- Comorbidities were determined using the Charlson Comorbidity Index (CCI). Significant comorbidities included poor kidney function and age greater than 65.
- Only 27% of patients had data on the critical status of recommended markers del(17p)/TP53 mutations. This is difficult to understand as Test Before Treat™ has been the standard of care for nearly a decade. Moreover, of the 73% of untested patients, only 44% received Ibrutinib, while received 32% chemoimmunotherapy (CIT), and 14% received monotherapy with anti-CD-20 agents like Rituximab. This means that the majority of these patients received therapies that were likely of little or no value.
- Of patients with the high-risk markers, del(17p)/TP53 mutations, about a quarter received non-recommended chemoimmunotherapy (CIT) such as Fludarabine, Cyclophosphamide, and Rituximab (FCR) or Bendamustine and Rituximab (BR). This is inexcusable as CIT is ineffective in this population. The NCCN recommended Ibrutinib.
Conclusions:
Too many patients with del(17p)/TP53 mutations, who are at high risk for poor outcomes with CIT, did not receive the NCCN-recommended treatment. In addition, most patients did not have the recommended testing for del(17p)/TP53 mutations (see Test Before Treat™) and thus may have received sub-optimal treatment.
Our motto is: Smart patients get smart care. Make sure your doctor evaluates you for high-risk factors, including del(17p)/TP53 mutations, and follows the NCCN recommendations for the best treatment for your CLL / SLL.
Please enjoy this interview with Dr. Barrientos from the virtual 2021 ASH meeting
Watch Dr. Koffman’s review of the abstract below:
You can read the actual ASH abstract Application of National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for CLL / SLL from the informCLL Real-World Registry here, where you will find more exciting details and analyses of how the various groups were treated which often conflicted with the recommended NCCN guidelines.
Be strong. We are all in this together.
Dr. Michael R. Green MD and CLL patient.