Introduction:
Various newer treatments have been developed for relapsed or refractory chronic lymphocytic leukemia / small lymphocytic lymphoma (CLL / SLL). The Murano study first presented results in 2017 and employed longitudinal assessments of minimal or measurable residual disease (MRD) to demonstrate the superior outcomes using fixed duration VenR compared with an older chemoimmunotherapy, bendamustine plus rituximab (BR). The techniques can detect as few as one cancer cell per 100,000 normal cells or 10-5, although the cut-off for undetectable (u)MRD was the standard 10-4.
Murano had already shown superior rates of undetectable (u)MRD and progression-free survival (PFS) in patients with relapsed/refractory CLL treated with fixed-duration VenR compared to BR.
At the 2021 annual meeting of the American Society of Hematology, CLL Society’s Medical Advisory Board Co-Chair Dr. Alex Danilov interviewed Dr. Arnon P. Kater, the lead author of this article and Chair of the Dutch CLL society, to discuss the evolving results of the Murano trial four years later. Please enjoy this interview and article about very hopeful treatment.
Takeaways:
- In the Murano trial, 284 patients with relapsed or refractory CLL / SLL were randomized into two treatment arms, consisting of fixed duration VenR (Ven 400 mg for two years combined with standard dose R for the first six months) or standard dose BR for six months.
- The Median follow-up was five years.
- This abstract looked only at a select group of patients who had deep responses to therapy. Specifically, only those patients that completed treatment without progressive disease (PD) and had at least 2 MRD measurements after the end of treatment (EOT) were eligible for evaluation.
- 91 patients in the VenR arm and 120 in the BR group were evaluated with EOT and post EOT MRD.
- The longitudinal assessment of MRD allowed for reporting the kinetics of MRD growth after EOT.
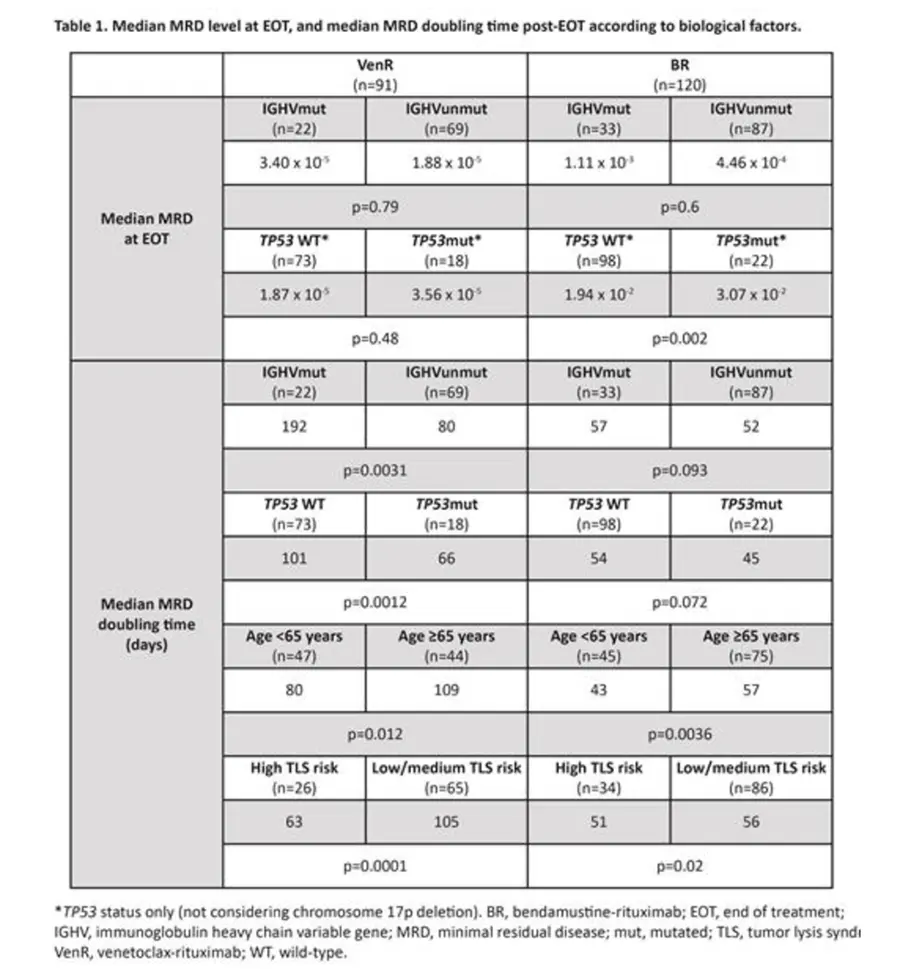
- The MRD levels in peripheral blood (PB) were tested every 3-6 months, with the threshold at less than 10-4. After EOT, the undetectable measurable residual disease or uMRD was significantly lower in the VenR group at 1.88 X 10-5 compared to 7.06 X 10-4 in the BR group. In other words, the depth of remission (amount of measurable residual disease) was almost 40-fold less in the VenR patients
- In the VenR group, the superior MRD doubling time was achieved at 91 days, compared to only 53 days in the BR group.
- The possibility of using MRD doubling time to predict prognosis is being studied.
- While unmutated IGHV and the presence of TP53 mutation are well established negative prognostic markers, fortunately, in this study, the presence or absence of these negative prognostic factors were shown to have no effect on MRD at EOT in the VenR group.
- However, unmutated IGHV, mutated TP53, age less than 65 years, and tumor burden at the beginning of treatment (potential for tumor lysis syndrome) all had a negative impact on the MRD growth rate after EOT. Regardless, VenR had an MRD growth rate post EOT that was 0.51-fold slower at a statistically significant level compared to the BR group.
Conclusions:
It is not surprising that the fixed duration, non-chemotherapy treatment of venetoclax plus rituximab (VenR), provides significantly deeper remissions at the end of treatment compared to the chemoimmunotherapy combination of bendamustine plus rituximab (BR). What is intriguing is that the MRD doubling time after treatment in the VenR group was significantly longer, suggesting the choice of therapy might not only affect the depth of remission but also the kinetics of how quickly the CLL returns. If confirmed in other studies and if it is proven to correlate with overall and progression-free survival, this would have significant implications for treatment choices and how trials are designed. Further, comparing treatment outcomes is much more accurate, using the recently improved measurement of MRD at 10-5, and can potentially provide better prognostic information for patients. By the way, the MRD testing in the blood was not done using the clonoSeq test but rather the older techniques of allele-specific oligonucleotide-polymerase chain reaction (PCR) and/or flow cytometry.
The Murano trial provides great hope for very deep and durable remissions for relapsed or refractory CLL / SLL patients, including those with TP53 mutation or unmutated IGHV.
Further, similar hope is found in the fixed duration Ven plus Obinutuzumab results in front-line treatment of CLL / SLL patients.
Closing
Please enjoy this interview with Dr. Arnon P. Kater from the virtual 2021 ASH meeting.
You can read the published abstract here: Chronic Lymphocytic Leukemia (CLL) Clonal Growth Rate Is Slower Following Venetoclax-Rituximab (VenR): Results from a Minimal Residual Disease (MRD) Model from the Randomized Phase 3 Murano Trial
For more data squeezed out of the Murano trial from last year’s ASH meeting, you might also enjoy this interview with its lead author, Dr. John Seymour: ASH 2021: Assessment of the Clonal Dynamics of Acquired Mutations in Patients (Pts) with Relapsed/Refractory Chronic Lymphocytic Leukemia (R/R CLL) Treated in the Randomized Phase 3 Murano Trial Supports Venetoclax-Rituximab (VenR) Fixed-Duration Combinat
Be strong. We are all in this together.
Dr. Michael R. Green MD and CLL patient.



