Here is your weekly summary of what has been happening this past week in the United States.
Weekly COVID-19 Statistics
According to the CDC’s map of community level of spread, 23.6% of the population lives in an area with medium to high transmission rates. This is a 2.11% increase from last week.
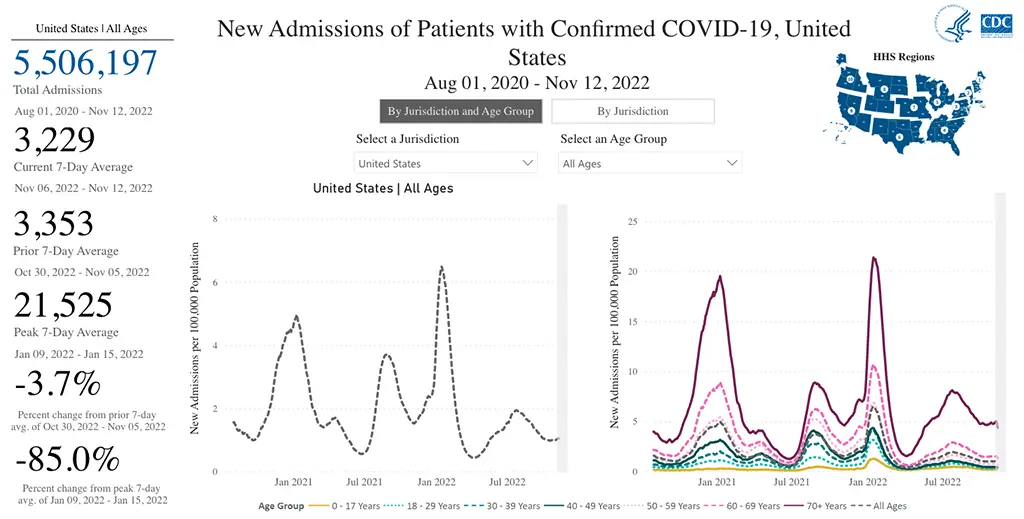
The rate of hospitalizations as of November 12th indicate that hospitalizations remain mostly flat, but are down overall by 3.7% from the prior week with the exception of some slight increases in the East and Midwest. There was also a slightly increased rate of hospitalizations in those over 70 when broken down by age.
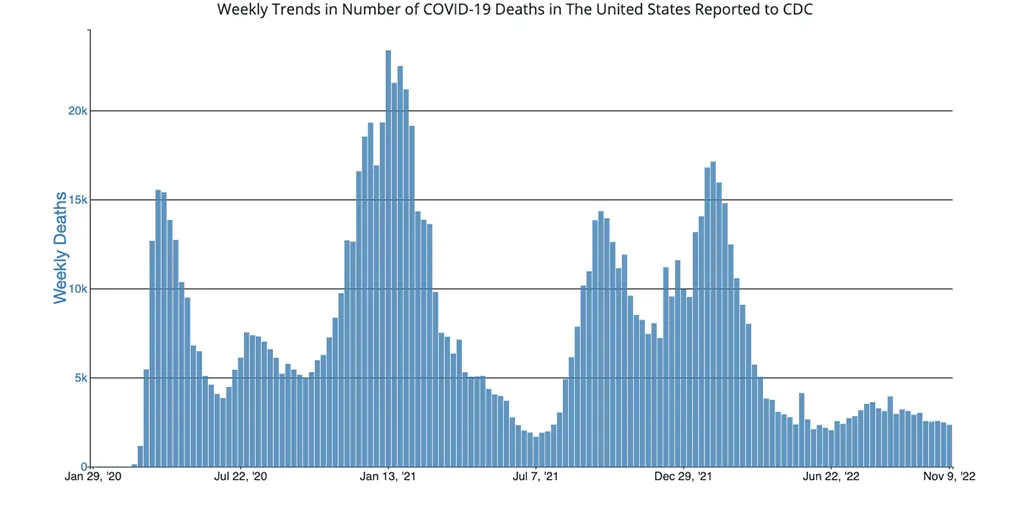
The number of deaths from COVID-19 in the US ending November 9th were 2,344, which is also down slightly from the week prior.
Current & Emerging Variants of Concern (VOC)
As suspected, the variants of concern that emerged quickly in the US a couple of weeks ago are outcompeting BA.5 at lightning speed. The relevance of this to the immunocompromised community (including all of those with CLL / SLL) is that BA.5 is the only remaining variant in the US that Evusheld still works well against. Here is an overall breakdown of US variants as of Friday, November 12th.
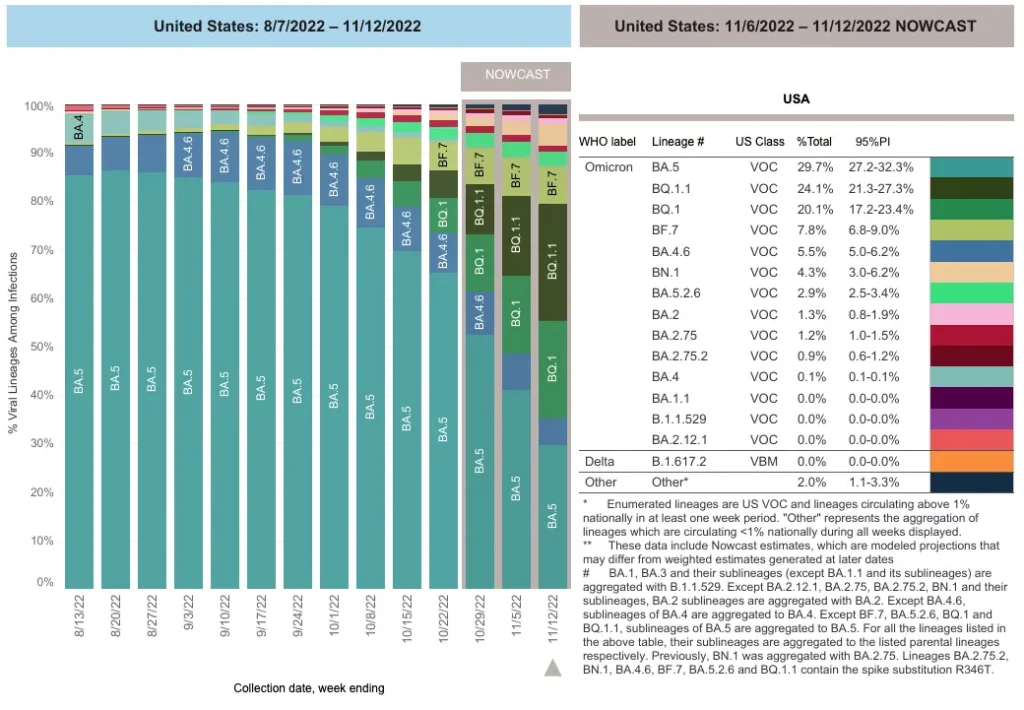
As if there were not already enough letters combined with numbers to describe all of these different variants the CDC is tracking (which some are now dubbing the “Scrabble” variants or the “alphabet soup variants“), one new one was added to the mix this week in the US. This new one is called the BN.1 variant, which now makes up 4.6% of cases on average. There is no confirmatory data regarding how BN.1 stacks up against our monoclonal antibody preventative (Evusheld) or treatment (Bebtelovimab) just yet. However, we can ascertain that it too will evade them both. The reason for this is that if you look at the small print located in the bottom right-hand side of the graphic above from the CDC, you will notice it states, “BN.1 was previously aggregated (or grouped in) with BA.2.75.” What we do know is that variants that offshoot or mutate from a previous variants (such as BA.2.75) that specifically contain the spike protein substitution called R346T do not hold up well against Evusheld (and sometimes Bebtelovimab). More confirmatory data will be sure to come if BN.1 begins to show a significant growth advantage in the US.
Here is a summary of what we know about the current variants of concern and how they hold up against Evusheld and Bebtelovimab as of the time this update was written:
Again, it is important to note that both Evusheld and Bebtelovimab are technically STILL CONSIDERED EFFECTIVE against the currently dominant variant of concern, BA.5 (30.3% of cases), against BA.2 (1.2% of cases) and BA.2.75 (also 1.2% of cases).
The preliminary data indicate that Bebtelovimab will no longer be effective against the following variants:
- BQ.1
- BQ.1.1
These two variants together made up 46.3% of cases nationwide as of November 12th.
The preliminary data indicate that Evusheld will no longer be effective against the following variants:
- BA.4.6
- BA.2.75.2
- BQ.1
- BQ.1.1
- BF.7
- BN.1
These six variants together made up over 63.4% of cases nationwide as of November 12th.
While the US average of BA.5 has now dropped to only 30.32% of cases, there are significant regional differences in the percentage/prevalence of all the variants of concern. So, it is of utmost importance to keep an eye on the percentage of variants in your region by watching the breakdown that is updated weekly on the CDC’s variant tracker.
For instance, you can see that BA.5 makes up less than 20% of COVID-19 cases in the upper Northeast part of the country, specifically New York state. By comparison, in the Midwest, BA.5 accounts for 44% of cases. So it is of utmost importance that everyone keeps an eye on the CDC’s COVID-19 Variant Proportion Data Tracker, which is updated every week on Fridays.
The most concerning variants in the US remain to be BQ.1 and BQ.1.1, since they are showing the most marked growth advantage over other variants, and both evade Evusheld and Bebtelovimab. Bebtelovimab is often reserved to treat those who are at high-risk of developing severe COVID-19 due to either not being able to take Paxlovid or whose symptoms are progressing despite already being on Paxlovid.
In New York state where BQ.1.1 is currently the highest in the US, it is encouraging that there is still no sign of increased hospitalizations (per the New York Times COVID Data Tracker).
It’s great news that Paxlovid and Remdesivir are still very effective antivirals and are holding up well against all current variants of concern. However, keep in mind that Paxlovid must be started within five days of symptom onset. So, test early and often if you have known exposure or symptoms occur (preferably obtaining a more sensitive PCR test) so you can qualify for these treatments should you test positive.
COVID-19 in the News This Week
President Biden extended the COVID-19 public health emergency declaration until at least mid-January in anticipation of the winter case surge. This is important because once the public health emergency is lifted, the federal government will stop paying for COVID-19 vaccines, tests, preventatives, and treatments, shifting the costs to the private sector.
An article was published in Nature examining the effects of those who are reinfected with COVID-19 showed that becoming infected more than once is not at all benign and should be avoided at all costs. There was evidence of an increased risk of developing one or more long-term health problems (including cardiovascular, hematological, endocrinological, gastrointestinal, musculoskeletal, neurological, and pulmonary-related symptoms) the more times a person has become infected, and the risk is cumulative. So the more times you become infected with COVID-19, the higher your risk of developing other conditions that can involve multiple organ systems. Even if you have had COVID-19 before and experienced what could be considered as “mild” symptoms, these data suggest there is a cumulative risk of developing long-term problems the more times you have been infected.
The FDA officially updated the Bebtelovimab Healthcare Provider Fact Sheet to include information regarding expected reduced activity against the BQ.1 and BQ.1.1 variants, specifically to let healthcare providers know that it will not neutralize virus caused by these variants.
CLL Society was quoted multiple times in this CNN article, “Many Patients with Weak Immune Systems Don’t Realize Their COVID-19 Medicine Isn’t as Effective as It Used to Be” which aimed to help get the word out to the immunocompromised (and the public at large) that Evusheld is no longer going to provide any protection here in a couple of weeks when BA.5 disappears in the US.
A new review on mRNA vaccines demonstrated the marked potential to adjust the mRNA-nanoparticle platform to improve delivery efficiency, durability of efficacy, and reduce the side-effects of future COVID-19 vaccines. The question is why this is not being aggressively pursued by the government and drug companies to develop next-generation COVID-19 vaccines which could be more effective, better tolerated, and potentially have at least one full year of durable protection (versus the current 3-4 months). Unfortunately, the answer to the question seems to be an overall lack of government funding.
In Summary
While the news this week is not at necessarily what we wanted to hear, as a community we all know exactly what to do! It’s time once again to mask up with a well-fitted N95 or KN95 mask any time you are in contact with individuals outside of your home, review your COVID-19 Action Plan, practice good hand hygiene, try to have good air-flow/ventilation whenever you are around others as much as possible (especially those who are unmasked), make sure you have received your bivalent COVID-19 booster (and encourage those in your household or other friends/family members you are in frequently in contact with to obtain it as well), and practice social distancing as much as possible. We will get through this!
Keep learning and stay well.
Robyn Brumble, MSN, RN
Director of Scientific Affairs & Research
CLL Society

















