As many of you know, there has been some big news for the immunocompromised community that happened this past week. Unfortunately, the FDA made the decision to discontinue the Emergency Use Authorization for Evusheld due to it being resistant to the vast majority of circulating variants in the US (>95% on average nationwide). CLL Society has been anticipating this decision coming for several months, but it has been disappointing news to officially receive this past week, nonetheless.
In light of the Evusheld news, the CDC is urging all of those who are immunocompromised (this includes everyone diagnosed with CLL / SLL, regardless of their treatment status) to take extra precautions to avoid COVID-19 infection and severe disease by doing the following:
- Obtain a bivalent booster.
- Always wear a high-quality KN95 or N95 face mask when in contact with others outside of your home. You can obtain quality N95 masks free of charge from many local pharmacies across the country.
- Practice social distancing and avoid poorly ventilated or crowed indoor spaces as much as possible.
- When indoors with others do everything possible to improve ventilation such as opening windows or using a HEPA air filtration system.
- Wash your hands and use hand sanitizer often.
- If any symptoms occur, obtain COVID-19 testing early and often, preferably with a PCR test, and call your healthcare provider.
- For those who are immunocompromised, getting a COVID-19 antiviral medication started as soon as possible is now of even more importance with Evusheld no longer providing an extra protective layer. Remember, Paxlovid must be started orally within five days of symptom onset and Remdesivir must be started intravenously within seven days of symptom onset.
These are all of the same exact things CLL Society has been urging since before the holidays!
Nitric Oxide Nasal Spray
As a result of our most recent COVID-19 webinar where Nitric Oxide Nasal Spray (NONS) was mentioned, we have received multiple inquiries surrounding its use. Currently, NONS is not approved by the FDA, so it is not available for purchase within the US or Canada. However, it has received approval for use after infection and is available over-the-counter in Israel, India, Germany, Thailand, Singapore, Nepal, South Africa, Indonesia, and Bahrain. Approval in these countries were based on a study published in a Southeast Asia version of the Lancet indicating use of NONS reduced the viral load in COVID-19 patients who had already become infected and decreased their average length of symptoms and length of time they continued to test positive by four days on average.
There is also an ongoing Phase 3 clinical trial currently recruiting participants (in Canada and Sri Lanka) that will evaluate the ability of NONS to prevent COVID-19. This trial builds upon an observational study that linked the use of NONS after known COVID-19 exposure to a 75% reduction in the likelihood of becoming infected. Little to no side effects have been noted with the exception of mild nasal irritation in a small percentage of individuals. It is important to note there are very little clinical data on the use of NONS for COVID-19 prevention, but there are data to support the need for further clinical studies on this subject.
Here is an FAQ from the Canadian-based company Sanotize who created the technology for the nasal spray for those who would like to read more.
Weekly COVID-19 Statistics
Thank you to those of you in our community who have expressed their gratitude for these Weekly COVID-19 Updates throughout the winter months. As we continue to see downward trends in all data points after the winter holiday surge, after next week’s update CLL Society plans to slow these COVID-19 Updates down to bi-weekly while continually assessing the need to continue them on a weekly ongoing basis.
Here is this week’s CDC’s map of community level of spread (this particular link is weighted based on hospitalizations and not the actual level of spread). And here is the link to the New York Times actual cases per capita map as pictured below. The New York Times data was last updated January 29th.
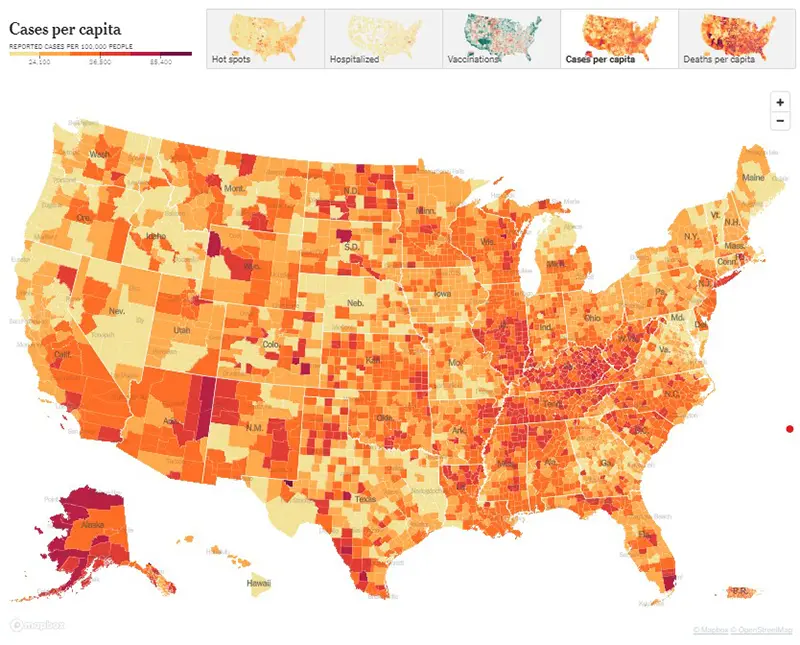
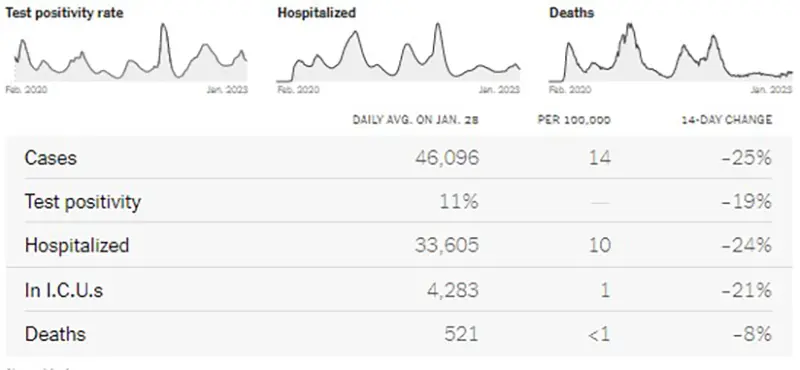
Here is the summary graphic from the New York Times as of January 29th. It is highly encouraging to continue seeing a downward trend in all 14- day change metrics.
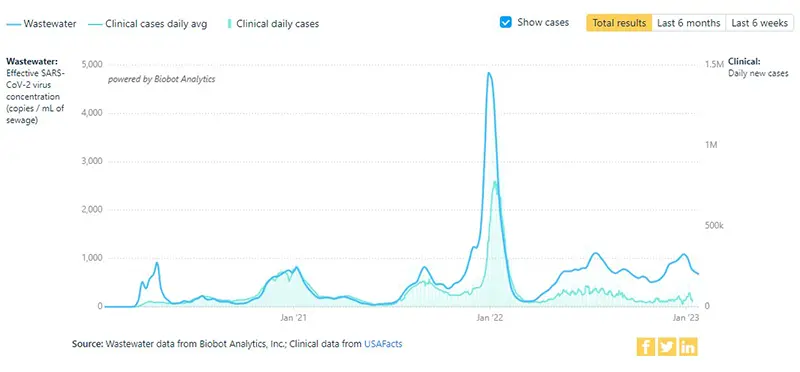
Here is a graphic from the Wastewater Monitoring Project last updated January 26th. This graphic verifies the downward trend in case numbers (as reflected above) is continuing and there are lower amounts of SARS-CoV-2 virus being detected in wastewater at this time compared to several weeks ago.
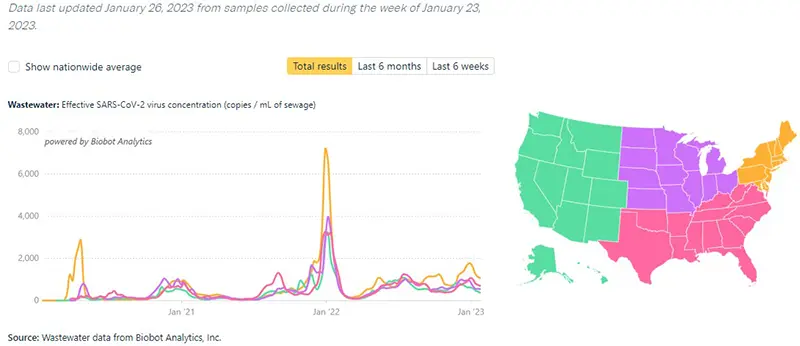
The additional graphic below from the Wastewater Project shows SARS-CoV-2 viral concentrations by region. All regions continue to reflect a downward trend, with the highest concentration of SARS-CoV-2 virus amounts detected in the Northeast region of the country.
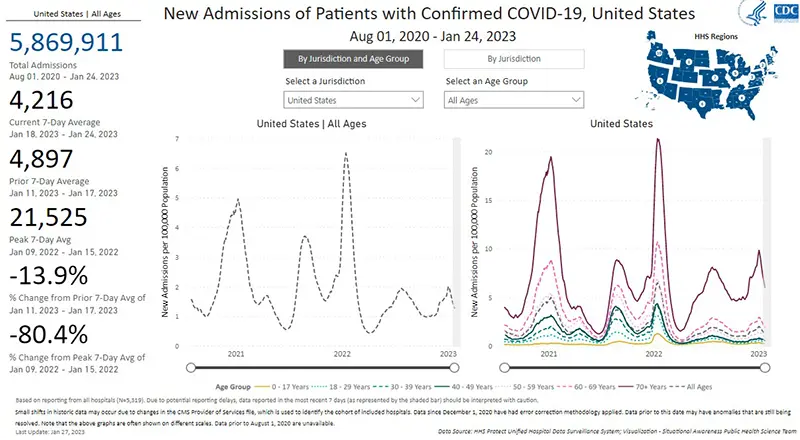
Hospitalizations due to COVID-19, last updated January 24th, continue to reflect a sharp decrease as well (gray dotted line graphic on the left). While there continues to be a contrast in the number of hospitalizations for those over the age of 70 as we have seen consistently since the winter wave began (purple solid line on the right), that age group is also experiencing a sharp decrease in hospitalizations week over week.
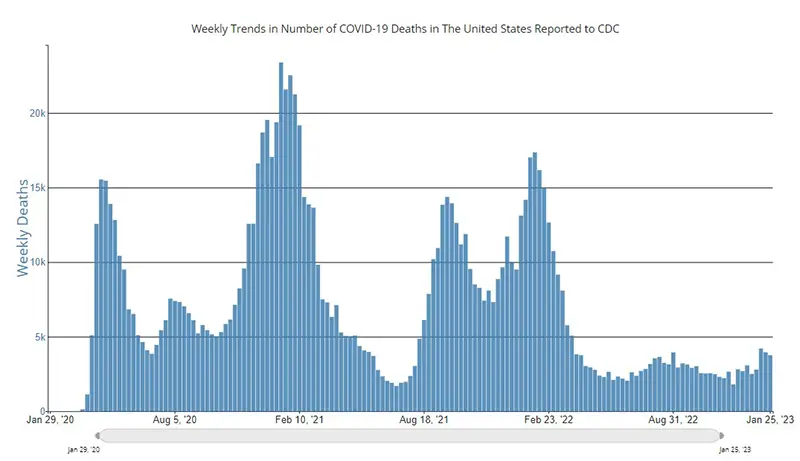
The weekly average number of COVID-19 deaths according to the CDC in the US ending the week of January 25th were 3,756, which is a decrease of 151 deaths on average from the week prior. We hope to continue seeing this downward trend in average weekly deaths, as this number is still not to the baseline seen prior to the holiday season.
Current & Emerging Variants of Concern (VOC)
Here is the overall breakdown of variants in the US according to the CDC’s Variant Tracker as of January 28th. You might notice a slight change in the way this graphic appears this week due to the CDC making some slight changes. As has been the case for many weeks now, the highly transmissible XBB.1.5 variant (also called the Kraken variant) continues to show significant growth advantage over all other variants circulating within the US.
Since BA.5, BN.1, BA.2, BA.2.75, and BA.4 are all but gone and the FDA pulled the Emergency Use Authorization for Evusheld this past week due to it being resistant to the majority of circulating variants in the US, we are no longer going to show the breakdown by region for BA.5.
COVID-19 in the News
- The FDA held their Vaccines and Related Biological Products Advisory Committee (VRBPAC) Meeting on January 26th. While many statistics were discussed about the bivalent vaccine and other COVID-19 vaccine related topics, the one thing the committee voted on was a new COVID-19 vaccination strategy that would simplify or streamline the type of vaccine everyone from this point forward receives. Before last week, those who had not received their primary series yet could only receive the two dose “priming” series of the monovalent (original formula) vaccine and then they were eligible for one dose of the bivalent booster. Now after the committee voted unanimously, those who are seeking COVID-19 vaccination for the first time will receive only the updated bivalent version of the vaccine for all priming and booster doses. There were additional conversations on when Americans would need more boosters, but there was no formal vote or decisions made. The committee proposed a meeting again in late May or early June of this year to discuss boosters for the fall, but did say that if another unexpected surge happened before then they were leaving the door open to reassess additional boosters before that time.
- Considering Evusheld has lost its Emergency Use Authorization from the FDA making the immunocompromised more vulnerable again to developing severe COVID-19 disease, the Department of Health and Human Services is in the process of updating their COVID-19 Therapeutics Locator tool to include outpatient infusion centers who have Remdesivir available added to the COVID-19 therapeutics locator tool. This will be a work in progress since Remdesivir has received full FDA approval, meaning it is commercially available and not being distributed by the government. So, at this time, the tool is completely dependent upon various outpatient infusion clinics notifying them that they have Remdesivir available for immunocompromised individuals who cannot receive Paxlovid for whatever reason.
- The CDC released a Morbidity and Mortality Weekly Report (MMWR) on January 25th with some good news about the mRNA bivalent booster’s effectiveness against preventing infection itself from the BA.5 variant and the XBB/XBB.1.5 sublineages. The researchers found that for adults aged 18 to 49 years, the relative vaccine effectiveness of a bivalent booster given two to three months earlier was 52% effective in preventing BA.5 infection, and 48% percent effective in preventing XBB/XBB.1.5 infection compared to those who received no bivalent booster.
In Summary
If you missed CLL Society’s most recent COVID-19 webinar last week, you can watch it on demand here.
Please know that CLL Society has remained diligent in working behind the scenes in terms of the ongoing policy and advocacy work that must continue on behalf of our immunocompromised community. As soon as the news on Evusheld was announced, we sent a joint letter that was signed onto by eight other patient advocacy organizations to key government officials. You can read more about the letter-writing effort here.
Keep learning, and please stay well.
Robyn Brumble, MSN, RN
Director of Scientific Affairs & Research
CLL Society

















