Authored by Dr. Brian Koffman
The Bottom Line:
Chronic lymphocytic leukemia (CLL) patients do better when their first therapy is acalabrutinib based compared to chemoimmunotherapy.
Specifically, after more than six years of follow-up, acalabrutinib (A) alone or in combination with obinutuzumab (A+O) had better progression free survival (PFS) and overall survival (OS) in all groups treated frontline for their chronic lymphocytic leukemia compared to obinutuzumab + Chlorambucil (O+Clb).
Who Performed the Research and Where Was it Presented:
Dr. Jeff Sharman and colleagues presented the results of six years of data from the Elevate-TN trial: Acalabrutinib ± Obinutuzumab Vs Obinutuzumab + Chlorambucil in Treatment-Naive Chronic Lymphocytic Leukemia: 6-Year Follow-up of Elevate-TN at the American Society of Hematology (ASH) Annual meeting in December 2023 in San Diego.
Background:
Previous reports have presented the superiority of acalabrutinib ± obinutuzumab versus obinutuzumab + chlorambucil, but this adds 16 months more of data.
Methods:
Untreated CLL patients who needed treatment were randomized to receive acalabrutinib alone or in combination with obinituzumab or obinutuzumab + chlorambucil. Those who progressed on O+Clb could cross over to acalabrutinib monotherapy.
Results:
- Patients:
- 535 patients were randomized: 179 to A, 179 to A+O, and 177 to O+Clb
- Median age was 70 years old.
- 63% had unmutated immunoglobulin heavy chain variable region genes (IGHV).
- 14% had del(17p) and/or TP53 mutation.
- Outcomes:
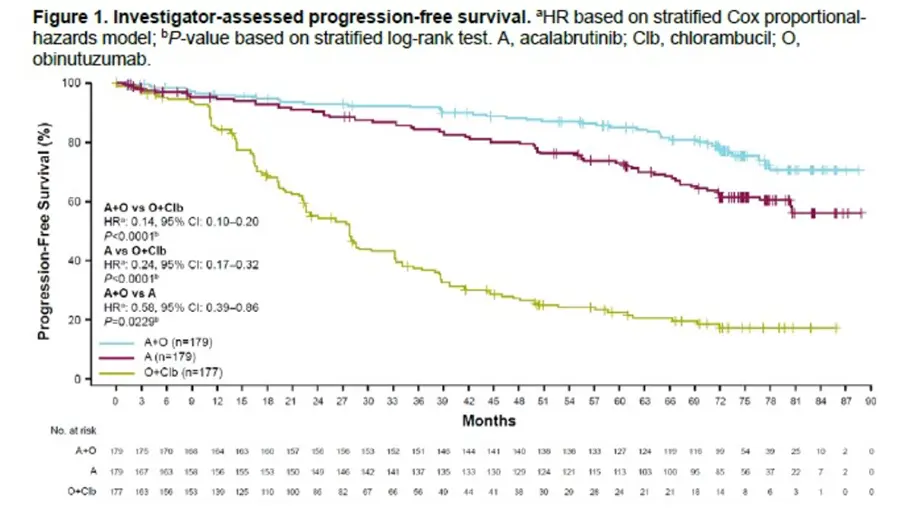
- At a median follow-up of 6 years follow-up, median PFS was not reached for A+O and A; median PFS was 27.8 months for O+Clb.
- Not surprisingly, the high risk 73 patients with del(17p) and/or TP53 mutation, median PFS was 73.1 months for A+O and NR for A but only 17.5 months for O+Clb.
- 79 patients who crossed over from O+Clb to A, their second PFS (time to second disease progression or death) was not reached.
- Estimated six-year OS rates were 84% for A+O, 76% for A, and 75% for O+Clb
- Adverse Events:
- Low neutrophil counts were seen in A+O and A in 31% and 12%, low platelets (8% and 3%), diarrhea (6% and 1%), COVID-19 (9% and 7%), pneumonia (7% and 6%), syncope (fainting) (5% and 2%), and hypertension (4% and 5%), respectively.
- Severe atrial fibrillation, hypertension, and secondary cancers were reported in 2%, 4%, and 10% of patients treated with A+O and in 2%, 5%, and 5% of patients treated with A, respectively.
- More patients discontinued treatment due to AEs 21% or 38 patients treated with A+O and 18% or 32 patients treated with A compared to progressive disease which was only noted in 6% or 10 patients treated with A+O and 14% or 25 of those treated with A.
Conclusions:
It should no longer be no surprise that a BTK inhibitor based therapy has once again proven superior to a chemotherapy based treatment. In fact, trials comparing targeted agents to obinutuzumab + chlorambucil would not be ethical to start now, as there would be no equipoise or balance in the trial. That was less clear when this trial started more than six years ago. It is encouraging to see the continued long-term responses to acalabrutinib ± obinutuzumab and not to see any new side effects emerge. However all CLL patients should take note of the 10% incidence of second cancers in those treated with acalabrutinib + obinutuzumab, likely the result of impaired immunity from the disease itself and its treatment.
Links and Resources:
Watch my monologue here:
To read the full ASH 2023 abstract that has more results and helpful graphics, please click on: Acalabrutinib ± Obinutuzumab Vs Obinutuzumab + Chlorambucil in Treatment-Naive Chronic Lymphocytic Leukemia: 6-Year Follow-up of Elevate-TN.
Stay strong. We are all in this together.
Brian Koffman MDCM (retired) MS Ed (he, him, his)
Co-Founder, Executive VP and Chief Medical Officer
CLL Society, Inc.

















