Patients with blood cancers, such as Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (CLL / SLL) are at high risk for breakthrough COVID-19 infection despite vaccination because of poor antibody response.
At the time of the development of this research and the reporting of its results, a third mRNA vaccine shot after the initial 2-shot series, was considered a “booster.” Since then, the preferred language is to refer to the third shot for the immunocompromised population as the final dose in the primary series and not a “booster”. To be consistent with the data as presented in the abstract, we will stick with calling the third dose a “booster” in this discussion.
Some patients have shown that an additional “booster” improved antibody response. A study is currently being conducted by the LLS National Registry (NCT04794387), evaluating 3300 patients with hematologic malignancies. As of July 2021, data on patients that received the “booster” were available for 24. Of these, 11 had CLL. Ages of all patients ranged from 51-79 years. Cancer treatment status and vaccine types were looked at with respect to antibody responses to the SARS-COV-2 spike protein. The Roche Elecsys assay was used to measure antibody response. This is the test used by LabCorp.
At the annual meeting of the American Society of Hematology (ASH) 2021, Dr. Brian Koffman interviewed Dr. Larry Saltzman, a Family Physician and Executive Research Director of the Leukemia and Lymphoma Society. They discussed the benefits of a “booster” vaccine dose for blood cancer patients.
Takeaways:
- Blood cancer patients did not respond to vaccines in 25% of cases reported in the LLS National Registry, with B-Cell malignancy patients at highest risk for being non-responders.
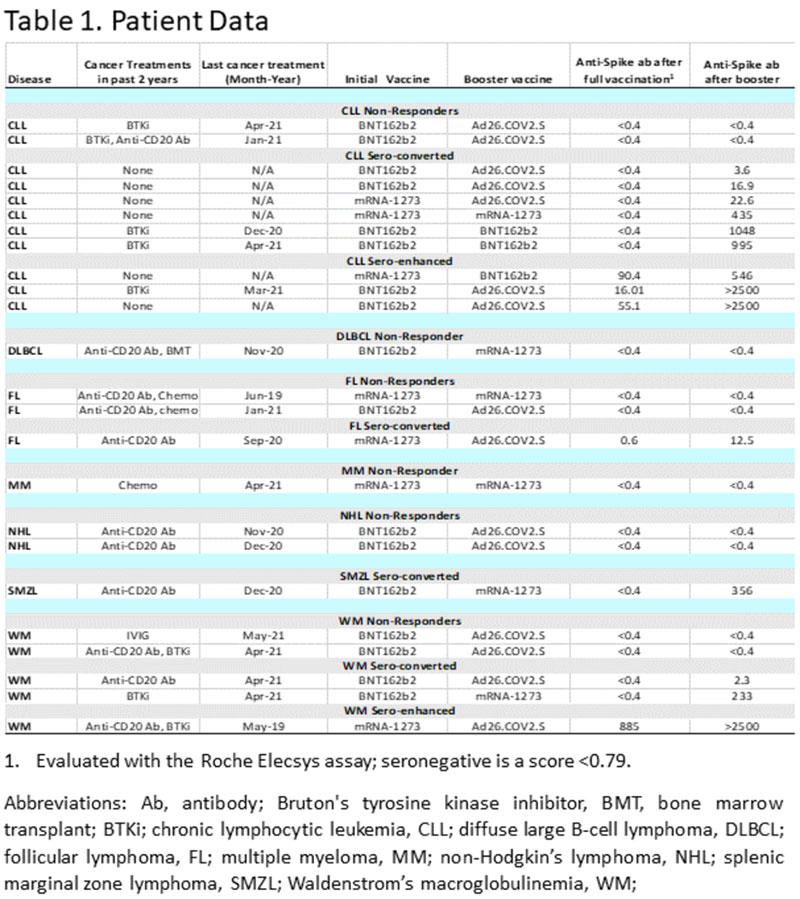
- As demonstrated in Table 1- of the patients who received a “booster”, only 3 of 11 CLL patients responded to the initial vaccine while an additional 6 responded to the “booster”, bringing the total to 9 of 11 that responded after the “booster”. Of the 3 that responded to the initial vaccine, all had a significant increased response to the “booster”.
- There were 2 of 11 CLL patients that had no response to the initial vaccine plus “booster”. One was the only CLL patient treated with an anti-CD20 Antibody (e.g., rituximab) plus a Bruton’s tyrosine kinase inhibitor (BTKi) in the past 2 years. The other non-responder was 1 of 4 CLL patients treated with a single-agent BTKi in the past 2 years. Both received mRNA BNT162b2 (Pfizer) for the initial 2 shots and the adenovirus Ad26.COV2.S (J&J) shot for the “booster”.
- Two of the 4 CLL patients treated with BTKi in the past 2 years had no response to the initial Pfizer vaccine but developed anti-spike antibody levels of 995 and 1048 after a Pfizer “booster”. The 4th patient that had been treated with BTKi in the past 2 years had a response of 16 after the initial Pfizer vaccine and a response of >2500 after a J&J “booster”.
- There were 6 CLL patients that had undergone no cancer treatment and only 2 responded to the initial vaccination. One received mRNA-1273 (Moderna) initial vaccination with a level of 90.4, and after Pfizer “booster” the level increased to 546. The 2nd of the 2 received Pfizer initial vaccination that resulted in a level of 55.1 with >2500 after a J&J “booster”.
- Four of the 6 untreated CLL patients had no initial response to either Moderna or Pfizer vaccines. Three of these received a “booster” with the J&J vaccine and achieved levels ranging from 3.6 to 22.6. The 4th patient received a Moderna vaccination followed by a Moderna “booster” and responded to a level of 435.
Conclusions:
Only 3 of the 11 studied CLL patients who received a “booster” produced a response to the first doses. A total of 9 patients of those 11 responded after a “booster” with anti-spike antibody levels ranging from 3.6 to >2500. There were 2 patients with no response even after receiving the “booster”, one treated with a single-agent BTKi and the other with BTKi plus anti-CD20 Antibody.
In the exciting interview with Dr. Saltzman, additional data on a much larger group of CLL patients are reviewed. Further, Dr. Saltzman briefly discussed the early data on T-cell response after vaccination and “boosters”. T-cells are another very essential contributing factor for protection from Covid -19 infection and outcomes.
The good news is that a third vaccine dose helps many with CLL / SLL to develop antibody responses. Unfortunately, we are still awaiting data that correlate antibody level to amount of protection.
You can read the abstract here: Sars-Cov-2 Antibody Levels in Blood Cancer Patients after a Third Sars-Cov-2 “Booster” Vaccination – Observational Data from the LLS National Registry
Please enjoy this video interview with Dr. Saltzman.
Stay strong, we are all in this together.
Dr. Michael R. Green MD, and CLL patient