Authored by: Dr. Brian Koffman
Bottom Line:
Fixed-duration glofitamab, a bispecific T-cell engager (BITE) monoclonal antibody, induces durable complete remissions (CR) and has a manageable safety profile in patients with Richter’s transformation.
Who Performed the Research and Where Was it Presented:
Dr. C. Carlo-Stella from Milan, Italy, led the presenters at the International Conference on Malignant Lymphoma (ICML) in June 2023 in Lugano, Switzerland.
Background:
Richter’s syndrome (RS) or Richter’s transformation (RT) are interchangeable names for when chronic lymphocytic leukemia / small lymphocytic lymphoma (CLL / SLL) transforms into an aggressive lymphoma, most commonly diffuse large B-cell lymphoma (DLBCL). It carries a dismal prognosis and remains a critical unmet need, with largely ineffective chemotherapy used as treatment. BITEs such as glofitamab have successfully treated similar aggressive lymphomas, and the hope was it might be similarly effective in RT. Glofitamab is a T-cell engaging bispecific antibody (Ab) with a novel 2:1 (CD20:CD3) format connecting to B cells that express CD20, including CLL and RT cells and T cells that express CD3 that do the actual killing.
Methods and Participants:
All patients had RT and received a prior regimen, including at least one anti-CD20 Ab, such as obinutuzumab or rituximab. Patients received obinutuzumab pretreatment (1000 or 2000mg) 7 days before the first glofitamab dose. As is common with BITEs, intravenous glofitamab was given either as a fixed dose or with step-up dosing (SUD) to reduce the risk of cytokine release syndrome (CRS).
Results:
- As of 10 October 2022, 11 patients had received glofitamab at a fixed dose (n = 5) or with SUD (n = 6).
- The median age was 71 years (range: 48–76).
- Overall, 91.0% of patients had Ann Arbor stage III–IV (advanced) disease.
- The median number of prior therapies was 3 (range: 1–4), and 54.6% of patients had ≥3 prior therapies.
- Most patients were refractory to a prior CD20 Ab-containing regimen (90.9%) and all to their most recent regimen (100%).
- 54.5% of patients were refractory to their initial therapy.
- Investigator-assessed overall response rate and complete response (CR) rates were 63.6% and 45.5%, respectively.
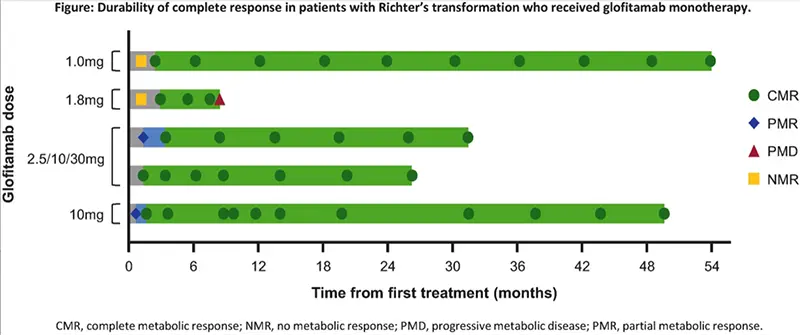
- The median time to CR was three months.
- Complete responses were durable; most (4/5; 80%) had been ongoing for ≥24.9 months at data cut.
- CRS occurred in 72.7% of patients, was primarily associated with the initial doses, and mainly was Grade (Gr) 1 (27.3%) or Gr 2 (27.3%).
- More severe Gr 3 (9.1%, n = 1) or Gr 4 (9.1%, n = 1) were uncommon.
- Glofitamab-related neurologic adverse events (AEs) potentially consistent with immune effector cell-associated neurotoxicity syndrome (ICANS) occurred in 5 patients (Gr 3, n = 1 [syncope]; Gr 1, n = 4).
- No glofitamab-related fatal AEs or glofitamab-related AEs leading to discontinuation were reported.
Conclusions:
Fixed-duration glofitamab given as monotherapy induces durable complete remissions in RT. CRS and ICANS are manageable. Glofitamab represents a promising therapy for patients with RT who need more options.
Links and Resources:
Watch Dr. Brian Koffman’s monologue on the abstract:
You can read the actual ICML abstract here: GLOFITAMAB MONOTHERAPY INDUCES DURABLE COMPLETE REMISSIONS AND HAS A MANAGEABLE SAFETY PROFILE IN PATIENTS WITH RICHTER’S TRANSFORMATION.
Stay strong. We are in this together.
Brian Koffman MDCM (retired), MS Ed
Co-Founder, Executive VP, and Chief Medical Officer
CLL Society

