Background:
Does the increased selectivity of the Bruton tyrosine kinase inhibitor (BTKi) acalabrutinib (Aca) compared to ibrutinib (Ib) improve tolerability while maintaining efficacy? The large, multi-center phase III ELEVATE-RR trial led by Dr. John Byrd, was designed to answer those questions for relapsed/refractory (R/R) chronic lymphocytic leukemia patients (pts). Results were presented at ASCO 2021 Annual Meeting, held virtually in June 2021.
Methods:
This was a non-inferiority trial which is a fancy way of saying the trial set out to prove that acalabrutinib worked at least equally as well as ibrutinib. Previously, treated CLL pts with del(17p) or del(11q) were randomized to receive either oral acalabrutinib 100 mg twice a day (BID) or ibrutinib 420 mg daily (QD).
The primary endpoint was progression-free survival (PFS).
Secondary endpoints of atrial fibrillation (AF), serious infection, Richter transformation, and overall survival (OS) were assessed.
Results:
- 533 pts (acalabrutinib, 268; ibrutinib, 265) were randomized.
- Median age was 66 and median prior therapies was two.
- It was tough to treat the group with del(17p) in 45.2% and del(11q) iin 64.2%.
- At a median follow-up of 40.9 months (range 0.0–59.1), acalabrutinib was noninferior to ibrutinib with a median PFS of 38.4 months in both arms.
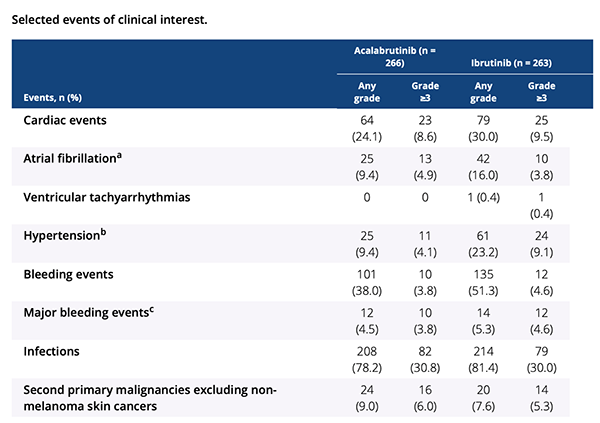
- Acalabrutinib was statistically superior to ibrutinib in all-grade AF incidence (9.4% vs 16.0%).
- Median overall survival (OS) was not reached in either arm.
- Among the other secondary endpoints, incidences of serious or grade ≥3 infection (acalabrutinib: 30.8%, ibrutinib: 30.0%) and Richter transformation (acalabrutinib: 3.8%, ibrutinib: 4.9%) were comparable between arms.
- Among all adverse events (AEs) seen in at least 20% of pts in either arm, acalabrutinib was associated with a lower incidence of hypertension (9.4%, 23.2%), arthralgia (15.8%, 22.8%), and diarrhea (34.6%, 46.0%), but a higher incidence of headache (34.6%, 20.2%) and cough (28.9%, 21.3%).
- AEs led to treatment discontinuation in 14.7% of acalabrutinib versus 21.3% of ibrutinib-treated pts. Certain side effects were of special interest: cardiac, hypertension, and bleeding events were seen less frequent with acalabrutinib.
Conclusions:
In this first head-to-head trial of BTKis in CLL, acalabrutinib was as effective as ibrutinib but with less cardiotoxicity and fewer discontinuations. Headaches were more common with acalabrutinib but these tend to diminish over time. This improved tolerance of acalabrutinib is important news, because as former Surgeon General, C. Everett Koop, famously once said, “Drugs don’t work if patients don’t take them.” We now know that we have two strong BTK options that provide high levels of durable CLL control in some of the most difficult to treat patients.
Here is the link to the ASCO 2021 abstract.
Please enjoy my video:
Stay strong. We are all in this together.
Brian Koffman MDCM (retired) MS Ed
Co-Founder, Executive VP and Chief Medical Officer
CLL Society, Inc.