INTRODUCTION:
Venetoclax is an oral treatment for chronic lymphocytic leukemia/small lymphocytic leukemia (CLL / SLL). Various combinations of venetoclax plus other agents such as venetoclax plus rituximab (RVe), venetoclax and obinutuzumab (GVe), or venetoclax plus ibrutinib plus obinutuzumab (GIVe) provide fixed duration treatment options with high rates of success.
Testing for undetectable measurable (or minimal) residual disease (uMRD) is a means by which the duration of treatment, success, and comparison of various treatment options can more accurately be determined. A consortium of researchers and facilities is simultaneously studying the success of various fixed duration treatment combinations using venetoclax and better ways of measuring uMRD. At the American Society of Hematology (ASH) 2021, Dr. Brian Koffman interviewed Dr. Stephen Stilgenbauer to discuss the findings.
TAKEAWAYS:
- The most common measurement of uMRD is done using 4-color flow cytometry (FCM). This method can identify less than 1 CLL cell among 10,000 lymphocytes (uMRD-4) or 10 to the minus four power.
- Using more sophisticated high-sensitivity flow cytometry FCM (hsFCM) methods, these researchers have been able to assess uMRD-5 and uMRD-6. The 5 or 6 refers to the number of zeros behind the number 1, where 5 represents 1 CLL cell in 100,000 lymphocytes, and 6 means 1 CLL cell in 1,000,000 (a million) lymphocytes. The higher the number following uMRD, the more sensitive or accurate the measurement is.
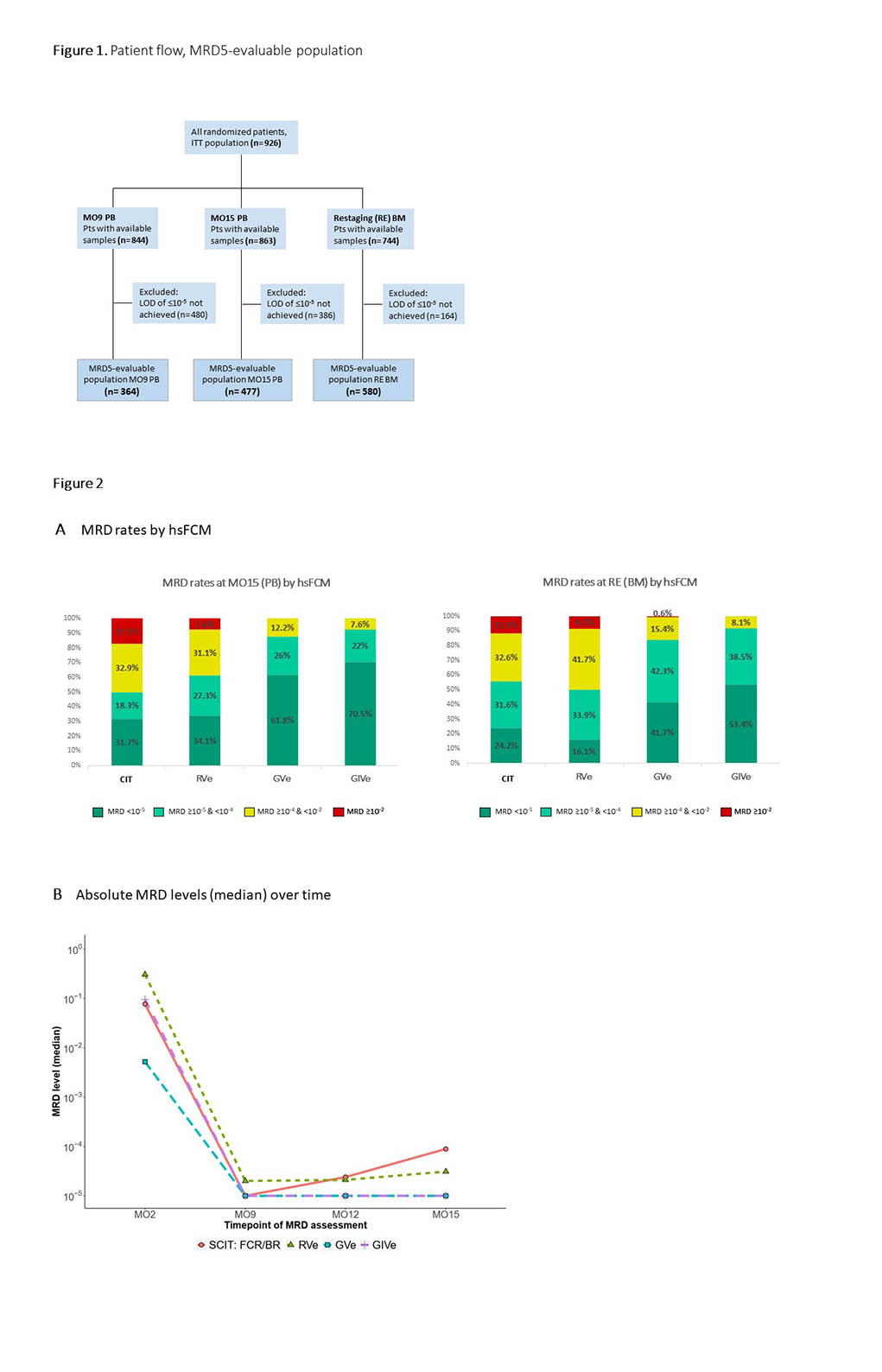
- In this phase 3 GAIA (CLL13) study, a total of 926 patients were randomized to receive one of four treatment options based on intention to treat (ITT).
- One group of 229 patients received a form of chemoimmunotherapy (CIT). The combination of fludarabine, cyclophosphamide, and rituximab (FCR) was used for fit patients under age 65, while bendamustine and rituximab (BR) were given to the unfit and/or over 65 year-olds.
- The other 3 arms in the trial received a finite duration of some combination of venetoclax, including 237 who were given venetoclax and rituximab (RVe), 229 venetoclax and obinutuzumab (GVe), and 231 venetoclax, obinutuzumab, and ibrutinib (GIVe).
- FCM measured the level of uMRD-4 at Month 2, Month 9, Month 12, and Month 15 in peripheral blood (PB) and in bone marrow (BM) at final restaging two months after the end of treatment
- Achievement of uMRD-4 at final restaging in bone marrow occurred in 37.1% of CIT, 43% of RVe, 72.5% of GVe, and 77.9% of GIVe patients.
- For many patients, samples for HsFCM testing were available at various points in both peripheral blood and bone marrow. In addition, the numbers of patients available for the testing median limit of detectable disease (LOD) in each of the 4 treatment arms at each point in time for both peripheral blood and bone marrow were similar. Therefore, the specific numbers are presented in the figures below.
- Among patients with peripheral blood samples available to evaluate with HsFCM at Month 15, uMRD-5 was achieved in 31.7% of CIT, 34.1% of RVe, 61.8% of GVe, and 70.5% of GIVe patients.
- Using HsFCM testing on bone marrow samples at final restaging, uMRD-5 was reached in 16.1% RVe, 24.2% CIT, 41.7% GVe, and 53.4% GIVe patients.
- The best correlation between HsFCM tested in peripheral blood versus bone marrow occurred in the venetoclax-containing treatments and was 60-70%, with GIVe having the highest correlation.
CONCLUSIONS:
The researchers in this study developed a superior measurement method of uMRD using a tool called HsFCM. It is now possible to determine uMRD-5 and uMRD-6 (1 CLL cell in 100,000 or 1,000,000 lymphocytes) compared to previous uMRD-4. This method allowed for a more accurate comparison of outcomes among 4 finite endpoint treatment combinations in CLL / SLL patients. The best results for uMRD-5 were achieved in treatments using a combination of venetoclax plus obinutuzumab or GVe. Adding ibrutinib as a third agent, or GIVe, was even better.
CLOSING:
Superior methods of detecting uMRD have been developed to guide patients and their doctors more accurately to determine the best treatment options for them and provide a better understanding of their prognosis and PFS.
SMART PATIENTS GET SMART CARE™. Be sure to ask your doctor about the best available, finite-duration therapies based on the measurement of uMRD-5 or uMRD-6 using the more sophisticated HsFCM. A promising option is the combination of venetoclax plus obinutuzumab with or without ibrutinib.
To read the actual ASH 2021 abstract, visit: High Resolution Assessment of Minimal Residual Disease (MRD) By Next-Generation Sequencing (NGS) and High-Sensitivity Flow Cytometry (hsFCM) in the Phase 3 GAIA (CLL13) Trial
Watch the video interview between Dr. Brian Koffman and Dr. Stephen Stilgenbauer below that discusses the evolving role of uMRD in trials and the clinic. They also discuss the various ways uMRD is measured:
Stay Strong, we are all in this together.
Michael Green MD and CLL patient
To learn more about uMRD and what it means for you, please look at this video: My MRD Journey
To learn more about fixed duration treatments with combinations that include Venetoclax and other agents like Ibrutinib, please go to:
or