Weekly COVID-19 Statistics
Here is the CDC’s map of community level of spread (this map’s risk is weighted based on hospitalizations), and here is the link to the New York Times actual cases per capita map as shown below. The New York Times data was last updated December 31st.
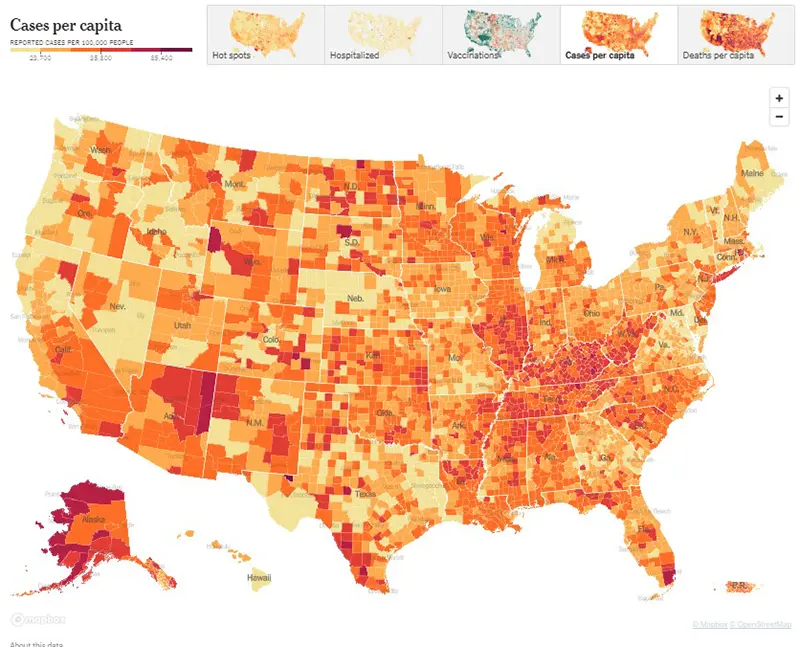
Here is the summary graphic from the New York Times. Again this week, there are going to be some gaps in the data due to decreased reporting around extended holiday weekends.
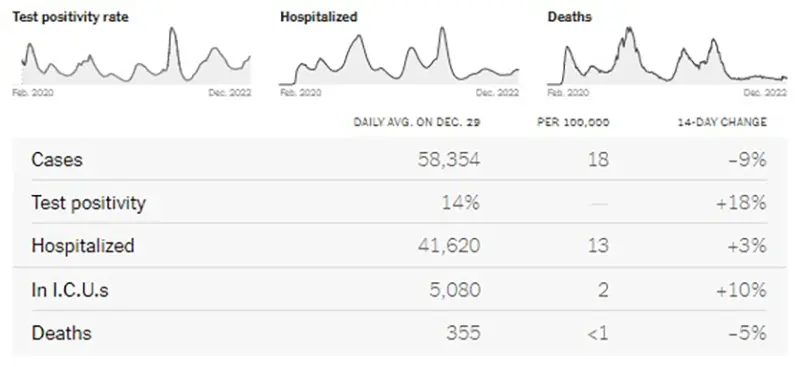
The COVID-19 Wastewater Monitoring Project, last updated December 29th, paints the most accurate picture we can have of true case numbers, as wastewater levels are not dependent upon people testing or health departments being open to report data. Note the top dark blue line (which is the amount of coronavirus measured in wastewater) continues to show a sharp increase. Compare this data point to the lower light blue line (which are the actual reported case numbers from health departments). This verifies that actual case numbers are much higher than what is being reported, just as we have seen historically since the Omicron wave in January 2022 when COVID-19 home testing kits became more readily available.
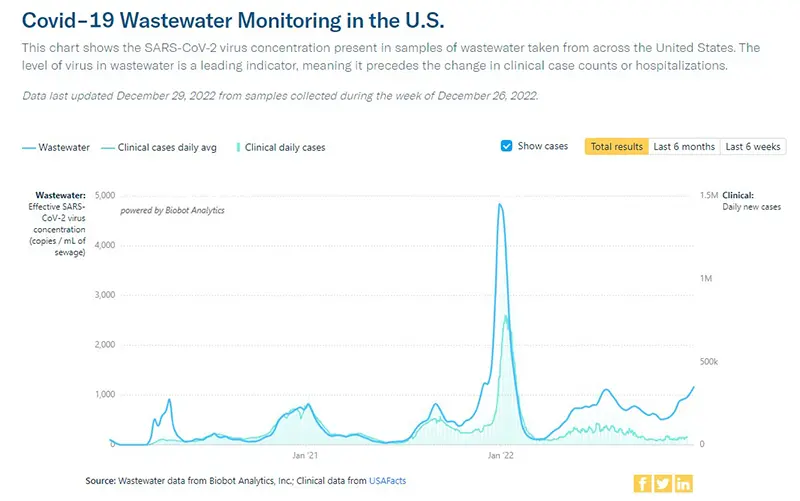
The rate of hospitalizations, last updated on December 28th, continue to indicate an increase in hospitalizations for COVID-19 in all age groups (gray dotted line graphic on the left). There continues to be a very concerning stark contrast in the number of hospitalizations for those over the age of 70 (dark purple solid line on the right).
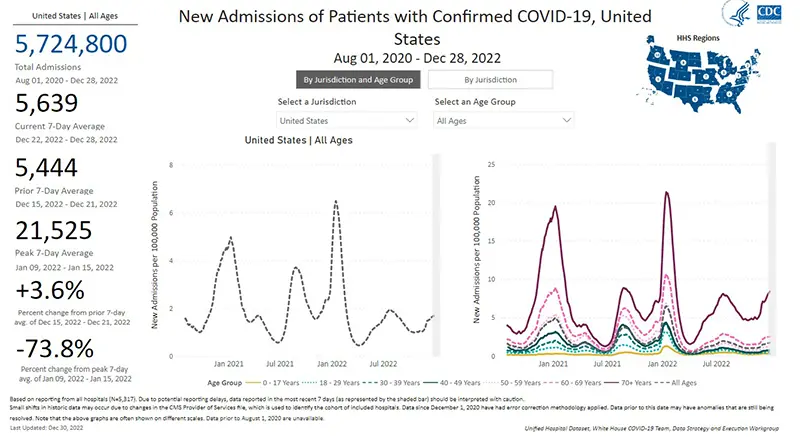
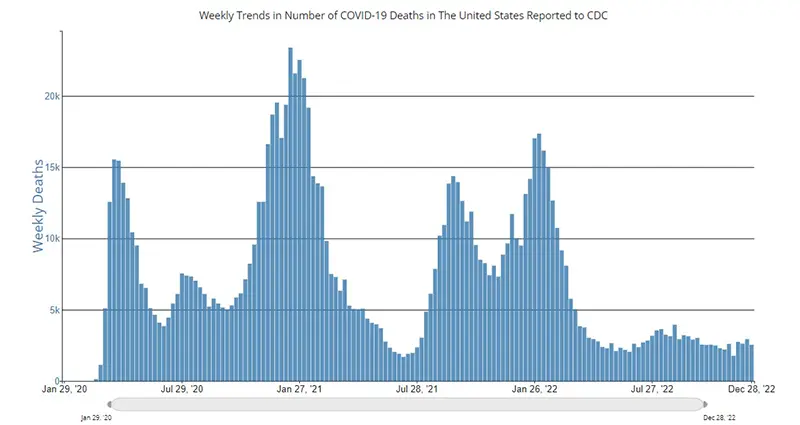
The weekly average number of COVID-19 deaths in the US ending the week of December 28th were 2,530. This is a decrease of 422 deaths from the week prior. Due to the holiday week(s) we often see a decrease in the reported numbers and can expect to see a rebound of those that were not reported in the following weeks. Of note, death rates have historically been a lagging indicator throughout the pandemic, meaning we typically see an increase in deaths after cases and hospitalizations increase approximately 2-3 weeks later.
Current & Emerging Variants of Concern (VOC)
If you have been following these weekly updates, you will notice a new bright purple variant representing XBB.1.5 has EXPLODED onto the variant tracker this week making up 40.5% of total cases! We mentioned in last week’s update that this would be a variant to keep an eye on. But we do not ever recall seeing a variant emerging and growing this fast in the US in the entire time CLL Society’s medical team has been monitoring these variants.
XBB.1.5 is a subvariant of the XBB variant (which was on the tracker last week and made up 18.3% of cases). XBB.1.5 is a “homegrown” variant that developed in New York State within the past month. The CDC has shared that they were aware of XBB.1.5 being around the past several weeks, but the numbers were being grouped in with the percentages of XBB. Now that it is outcompeting all the other variants including XBB, they are tracking it separately as a variant of concern independent of its predecessor.
At this rate, we can expect BQ.1 and BQ.1.1 to nearly disappear very soon. Sadly, Evusheld does not retain activity against XBB.1.5 either, but it is thought that the bivalent booster still provides protection against it.
Also of note, BF.7 is the variant causing cases to explode by the millions (yes millions) each day in China right now. It has not taken off quite yet here in the US (although has been detected), but it will be an interesting one to watch. Experts are also predicting there to be an even more infectious subvariant of BF.7, as we know the more opportunity that a variant has to spread the greater the chance (and speed) of there to be mutations from that particular variant. Some may remember in the early days of the pandemic we would talk about how one person on average would infect 3-5 other people. For reference, for each person who becomes infected with the BF.7 variant, they infect 18 other people on average. So, BF.7 itself is incredibly infectious. You may have heard several countries are again requiring testing of anyone who is traveling into their countries from China. This is the reason why.
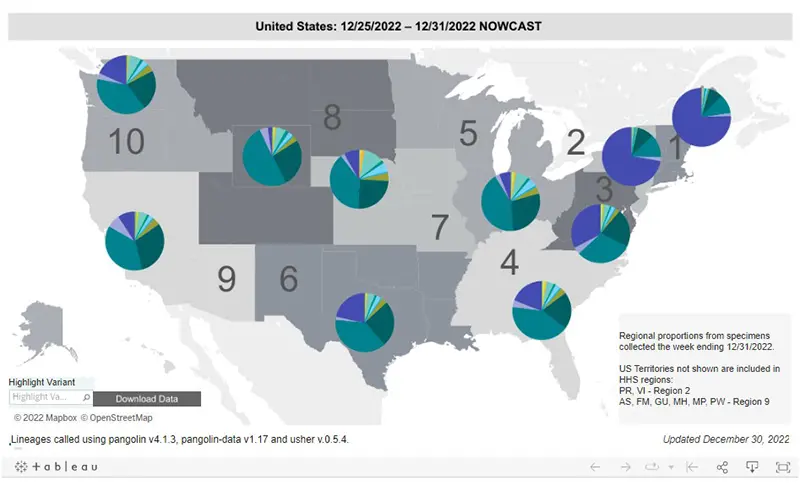
Here is the overall breakdown of variants in the US according to the CDC’s Variant Tracker as of December 31st.
And here is the breakdown by regions within the United States as of December 31st. You can see how striking of a difference there is in the amount of purple (XBB.1.5) in the East Coast regions of the US compared to the Midwest and West Coast.
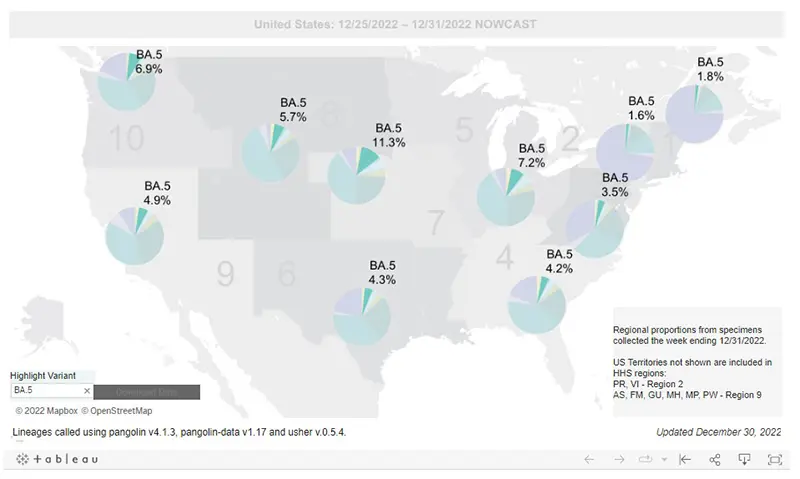
Since we have been sharing the regional amounts of BA.5 for all the previous updates, we will include it here again this week only because it is the main variant for which Evusheld still works against. However, you can see from this regional graph of BA.5 below that the percentage of cases that Evusheld will neutralize are miniscule at this point, which is why most academic institutions across the US are no longer offering to administer it.
Data indicate Evusheld will still provide protection against the following variants (percentages are national averages). Note that these overall percentages of variants that continue to have neutralizing capacity against the virus continue to decrease week over week:
- BA.5 (3.6%)
- BN.1 (3.8%)
- BA.2.75 (0.9%)
- BA.2 (0.3%)
These four variants together make up ~8.6% of cases on average nationwide as of December 31st.
Please discuss any questions you might have surrounding Evusheld with your healthcare provider.
Data indicate that Evusheld will NOT be effective against the remainder of variants in the US that are not mentioned specifically as the above four variants. (We are no longer going to list each of the variants that are not neutralized by Evusheld, as they are numerous and growing).
While we are extremely saddened that Evusheld no longer provides an additional layer of protection for all of those in our CLL / SLL community who are immunocompromised, we were encouraged to learn this week that the global clinical trial for what we could refer to as Evusheld 2.0 (for practical purposes) but will be actually called AZD5156 has gotten underway outside of the US. This clinical trial is named SUPERNOVA and we can expect clinical trial sites to be opening up in the US sometime in early 2023. Unfortunately, AstraZeneca is not expecting AZD5156 to become available outside of clinical trials until the fourth quarter of 2023. CLL Society will be sure to keep everyone informed as more information becomes available.
COVID-19 in the News
- An interesting preprint article was made available on an interesting trial that may indicate there could be a medication that can help prevent Long-COVID from developing. The trial looked at about 1100 patients who had become infected with COVID-19 and were randomized to receive either placebo, metformin, fluvoxamine, or ivermectin. They then monitored these same patients for 300 days, and what they found was that fluvoxamine and ivermectin did nothing to help prevent Long-COVID. However, those who received metformin had a 42% risk reduction in developing Long-COVID! That is a huge risk reduction. Remember that since this is a pre-print, this data still needs to be peer reviewed and we will still need large clinical trials to substantiate these findings. But metformin is an inexpensive oral diabetes drug that is used very commonly and is generally well tolerated, so this could be very exciting if further trials can replicate these findings.
- The results of a Phase 3 clinical trial were published in the New England Journal of Medicine looking at a new antiviral medication (VV116) that was developed in China and comparing it to Paxlovid in treating COVID-19. Patients were given a five-day course of either this new medication or Paxlovid. None of the trial participants experienced hospitalization or death and they recovered in a similar amount of time. The interesting difference between the two antiviral medications was that those who received VV116 had 10% fewer side effects reported compared to those who received Paxlovid. It is nice to see that there could potentially be another oral antiviral treatment for COVID-19 on the horizon.
In Summary
If you missed it last week, please read about the FDA’s recently updated COVID-19 testing recommendations here. Please remember, if you have known exposure to COVID-19 or are experiencing any symptoms at all, please get tested early, preferably with a PCR test, and call your healthcare provider. Paxlovid must be started orally within five days of symptom onset and Remdesivir must be started intravenously within seven days of symptom onset.
We encourage everyone to obtain your COVID-19 bivalent booster if you are eligible and have not yet done so. The FDA has a planned meeting after the first of the year to discuss recommendations for another booster, as we are approaching the four-month mark since they first became available to the public. We will update everyone as more information becomes available.
Please wear a well-fitted N95 mask (or KN95) while around others who live outside of your household. You can obtain quality N95 masks free of charge from many local pharmacies across the country.
Practice good hand washing often and use hand sanitizer, remember social distancing and avoid large indoor gatherings or crowded situations as much as possible, and try to ensure there is good air-flow and ventilation whenever you are around others by opening windows or doors as weather allows and/or using a quality HEPA air purifier if you have one available.
Please don’t miss CLL Society’s upcoming COVID-19 webinar in a couple of weeks. You can find more information and register for the webinar here.
Keep learning, and please stay well.
Robyn Brumble, MSN, RN
Director of Scientific Affairs & Research
CLL Society



