The Bottom Line:
Patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) who achieved undetectable measurable (or minimal) residual disease (uMRD) after fixed-duration ibrutinib + venetoclax treatment continue to have excellent outcomes four years after stopping initial therapy. The results of this study suggest that if patients achieve uMRD remission, it is safe to stop treatment.
Who Performed the Research and Where Was it Presented:
Dr. John Allan from Weill Cornell Medicine and colleagues presented the results at the American Society for Hematology Annual Meeting in 2022.
Background:
MRD measures how much disease is circulating in the blood or bone marrow. If your MRD test can’t detect any CLL cells, you have undetectable MRD (uMRD), which is excellent. We have more detailed information on MRD available here. Researchers are increasingly interested in using MRD status to guide treatment decisions (i.e., treat until reach uMRD), but clinical trial data is still needed to support this approach.
In this video, Dr. Deborah Stephens, a Director of the CLL program at the University of Utah Huntsman Cancer Institute, interviewed Dr. John Allan, a CLL specialist at Weill Cornell Medicine in New York City. They discussed recent results from the CAPTIVATE trial testing the combination of ibrutinib + venetoclax and MRD-guided discontinuation.
Methods and Participants:
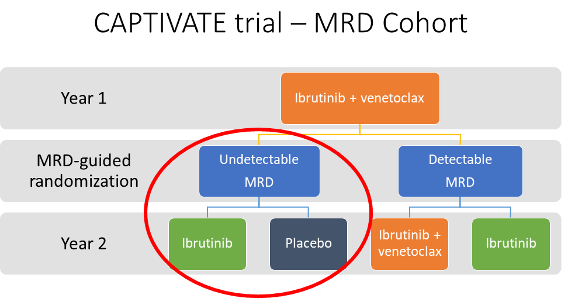
The CAPTIVATE study is a phase II clinical trial of ibrutinib + venetoclax for patients ≤70 years of age who had not previously been treated for CLL. The study design is summarized in the figure below, and the cohort discussed in this interview is circled in red. After one year of treatment with ibrutinib + venetoclax, 86 patients had undetectable MRD and were randomized to ibrutinib-only treatment or placebo (43 in each group).
Results:
- The CAPTIVATE trial has been going on long enough now that it has been about 3-4 years since patients stopped the initial ibrutinib + venetoclax therapy.
- At three years, disease-free survival (meaning no measurable disease detected) was 85% in the placebo group and 93% in the ibrutinib group.
- At four years, the progression-free survival (meaning the disease had not started growing again) was 88% in the placebo group and 95% in the ibrutinib group.
- At four years, the overall survival (meaning how many patients are still alive) was 100% in the placebo group and 98% in the ibrutinib group.
- These are great results for both groups. However, while ibrutinib may have a slight statistical progression-free advantage, it may not be meaningful considering the added side effects and costs of staying on therapy.
Conclusions:
Patients who achieved uMRD after fixed-duration ibrutinib + venetoclax treatment continue to have great outcomes four years after stopping initial therapy. The results of this study suggest that if patients achieve uMRD remission, it is safe to stop treatment. Using combination therapies to get patients to uMRD could benefit patients who don’t want to be on continuous therapies.
Links and Resources:
Watch the interview on the abstract here:
You can read the actual ASH abstract here: Treatment Outcomes after Undetectable MRD with First-Line Ibrutinib (Ibr) Plus Venetoclax (Ven): Fixed Duration Treatment (Placebo) Versus Continued Ibr with up to 5 Years Median Follow-up in the CAPTIVATE Study
Take care of yourself first.
Ann Liu, PhD

















