Weekly COVID-19 Statistics
Here is the CDC’s map of community level of spread (this map’s risk is weighted based on hospitalizations), and here is the link to the New York Times actual cases per capita map as shown below. The New York Times data was last updated January 8th.
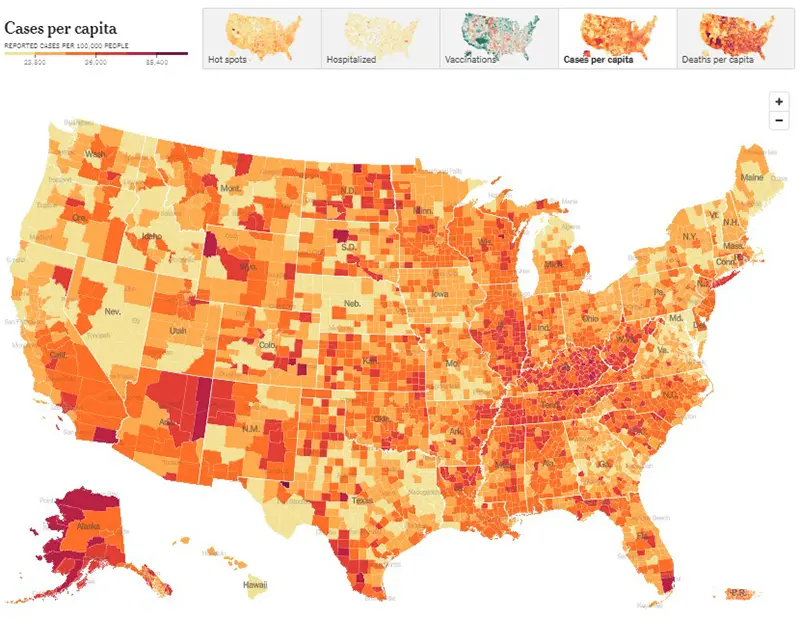
Here is the summary graphic from the New York Times as of January 8th. This summary indicates that hospitalizations, test positivity, and deaths are all rising at the national level, and reported cases have also begun to tick up modestly after a holiday lull. These metrics remain far below their all-time highs, but the growth in hospitalizations is concerning. According to the New York Times, around 47,000 people are currently hospitalized with COVID-19, which is the highest number seen since March of 2022.
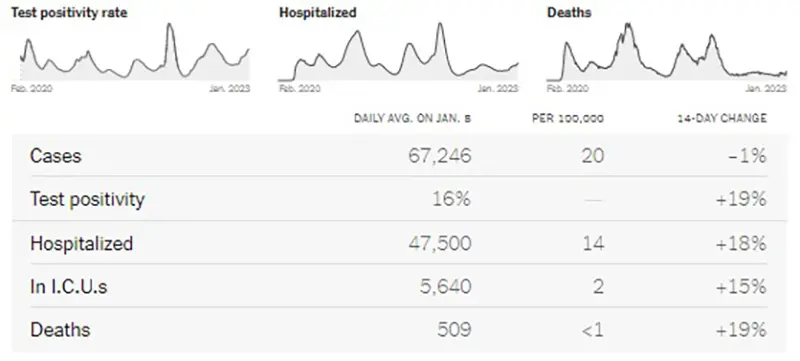
The COVID-19 Wastewater Monitoring Project data, last updated January 5th, paints the most accurate picture we can have of true case numbers, as wastewater levels are not dependent upon people testing or health departments reporting data. It appears that there may be a slight plateau this week, which would be great news after individuals gathered for the New Year holiday. Note the top dark blue line (which is the amount of coronavirus measured in wastewater) continues to show a sharp increase. Compare this data point to the lower light blue line (which are the actual reported case numbers from health departments). This verifies that actual case numbers are much higher than what is being reported, just as we have seen historically since the Omicron wave in January 2022 when COVID-19 home testing kits became more readily available.
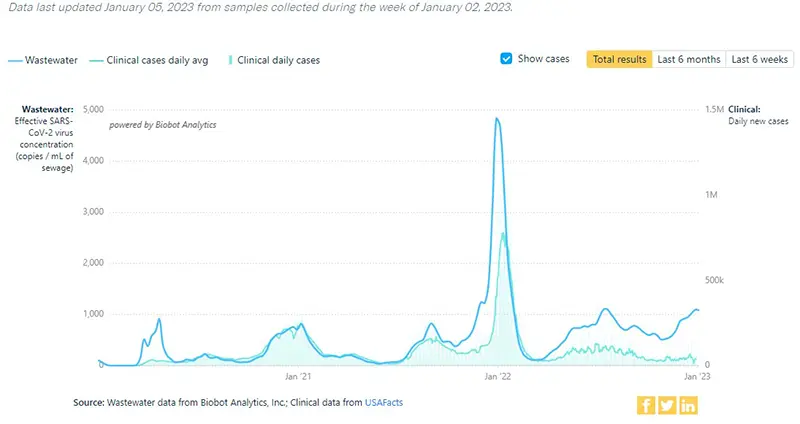
Here is an additional graphic from the Wastewater Monitoring Project that shows increases in viral concentrations by region. You can see that the area experiencing the sharpest increase in numbers right now is the Northeast where the variant XBB.1.5 has taken a hold.
The rate of hospitalizations, last updated on January 6th, continue to increase for all age groups (gray dotted line graphic on the left). There continues to be a very concerning stark contrast in the number of hospitalizations for those over the age of 70 (purple solid line on the right).
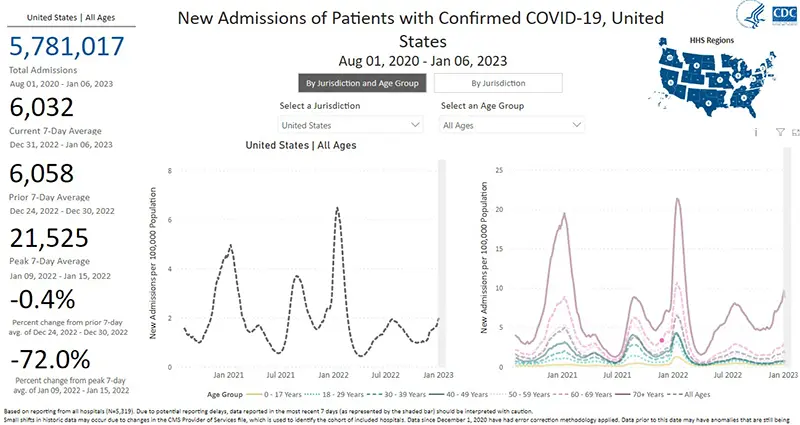
This week, we are including a graphic that clearly depicts the breakdown of hospitalizations in the Northeast region of the US where the New York state “homegrown” variant XBB.1.5 has taken a hold. While we don’t see a sharp increase overall throughout the country just yet, we can see that in the areas where XBB.1.5 is most prevalent, hospitalizations are especially rising in those areas as a result.
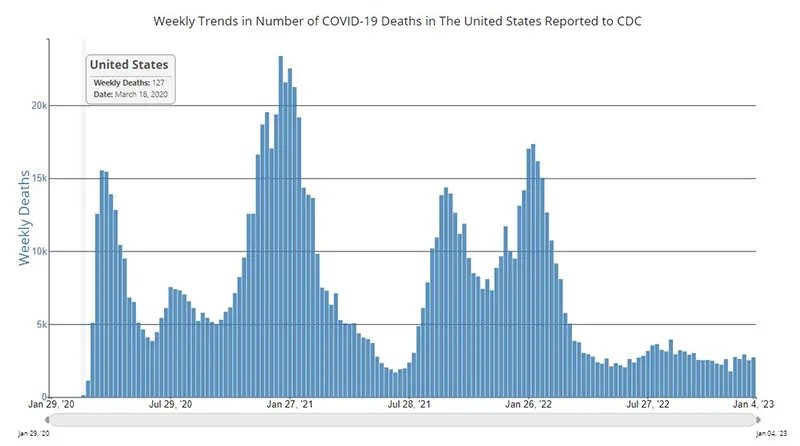
The weekly average number of COVID-19 deaths in the US ending the week of January 4th were 2,731. This is an increase of 201 deaths from the week prior. Of note, death rates have historically been a lagging indicator throughout the pandemic, meaning we typically see an increase in deaths approximately 2-3 weeks after we see increases in case numbers and hospitalizations.
Current & Emerging Variants of Concern (VOC)
As mentioned in last week’s COVID-19 Weekly Update, XBB.1.5 continues to outcompete both BQ.1, and by next week will most likely outcompete BQ1.1. Preliminary data indicate Evusheld does not neutralize XBB.1.5 either, but the bivalent booster still provides protection against it. In a FDA press release on January 6th they warned that Evusheld most likely does not protect against XBB.1.5. Also, many infectious disease experts are sounding the alarm that XBB.1.5 is one of the most infectious variants we have seen yet (which is why we are seeing it outcompete all of the other variants so quickly). This higher degree of infectiousness is in part thought to be due to acquiring a critical double mutation that maintains a high level of immune escape while increasing infectivity. What this means is XBB.1.5 has the ability to evade immunity developed from previous COVID-19 infections and it has a unique ability to bind even more tightly to receptors in the body than its predecessors.
Here is the overall breakdown of variants in the US according to the CDC’s Variant Tracker as of January 7th.
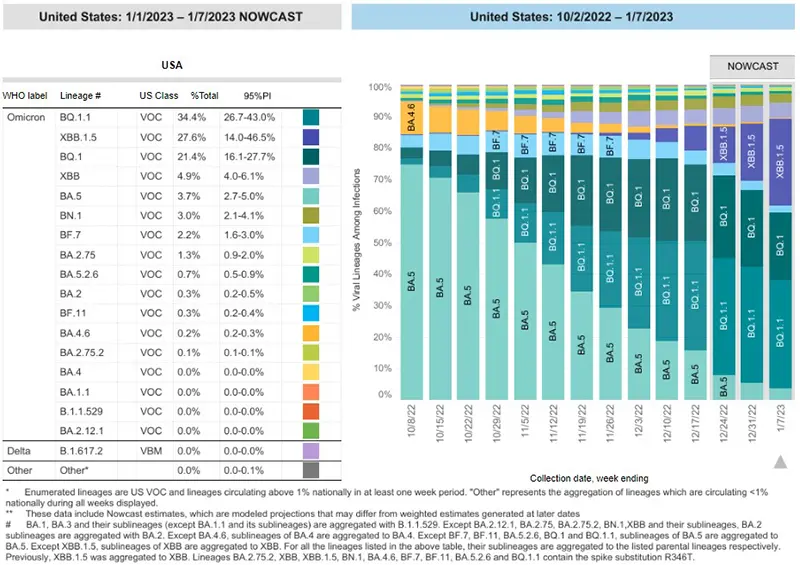
And here is the breakdown by regions within the United States as of January 7th. We are showing the proportion of BA.5 that remains by region, as this is the only variant that is still neutralized by Evusheld. While BA.5 makes up only 1-2% of cases in the upper Northeast regions of the country, it still makes up 11.9% of cases in the US (which still is not great in terms of Evusheld protection, but better all other parts of the country).

Data indicate Evusheld will still provide protection against the following variants (percentages are national averages). Note that these overall percentages of variants that continue to have neutralizing capacity against the virus continue to decrease week over week:
- BA.5 (3.7%)
- BN.1 (3.0%)
- BA.2.75 (1.3%)
- BA.2 (0.3%)
These four variants together make up ~8.3% of cases on average nationwide as of January 7th.
Please discuss any questions you might have surrounding Evusheld with your healthcare provider.
Data indicate that Evusheld will NOT be effective against the remainder of variants in the US that are not mentioned specifically as the above four variants. (We are no longer going to list each of the variants that are not neutralized by Evusheld, as they are numerous and growing).
COVID-19 in the News
- According to a pre-print study from Israel, the bivalent booster was found to reduce hospitalizations in those age 65 and older by 81% compared to those who had previously received at least two COVID-19 vaccinations (but not the bivalent). The study was carried out from the end of September until mid-December and looked at 622,701 people aged 65 and over who were eligible for the bivalent booster. Among them, 85,314, or 14%, had received it. Death occurred due to COVID-19 in only one of those who had received the bivalent booster and in 73 individuals who had not. Keep in mind that released pre-print articles still need to be peer reviewed, but this would be some of the first clinical evidence of the bivalent booster vaccine’s real-world effectiveness.
- The National Institutes of Health (NIH), in collaboration with the Administration for Strategic Preparedness and Response (ASPR) at the U.S. Department of Health and Human Services, has launched their pilot At Home Test to Treat Program, an entirely virtual community health intervention that will provide free COVID-19 health services including at-home rapid tests, telehealth sessions, and at-home antiviral treatments in selected communities to begin with. The program, first announced by the White House in September 2022, will make antiviral treatments available for eligible individuals who receive a positive test result, which could prevent severe illness, hospitalization or death. The program is being first launched in rural Pennsylvania, and one of the goals is to assist those who do not have equitable access to COVID-19 testing and timely treatments.
In Summary
If you missed the article that discusses the FDA’s most recent guidance on COVID-19 testing recommendations, you can read more here. Please remember, if you have known exposure to COVID-19 or are experiencing any symptoms at all, please get tested early, preferably with a PCR test, and call your healthcare provider. Paxlovid must be started orally within five days of symptom onset and Remdesivir must be started intravenously within seven days of symptom onset.
We encourage everyone to obtain your COVID-19 bivalent booster if you are eligible and have not yet done so. The FDA has another vaccine advisory committee meeting planned on January 26th to discuss future vaccination strategies for COVID-19. We might expect an additional recommended bivalent booster for the immunocompromised at that time, as we are approaching the four-month mark since they first became available to the public. We will update everyone as more information becomes available. If you would like to leave a public comment for the committee, reminding them to please keep the vaccination needs of the at-risk immunocompromised community in mind, especially now that Evusheld provides extremely minimal protection and Bebtelovimab is no longer an available treatment option, you can do so on the FDA’s public comment docket for this meeting.
Please wear a well-fitted N95 mask (or KN95) while around others who live outside of your household. You can obtain quality N95 masks free of charge from many local pharmacies across the country.
Please don’t miss CLL Society’s upcoming COVID-19 webinar that is being held this week! You can find more information and register for this webinar here.
Keep learning, and please stay well.
Robyn Brumble, MSN, RN
Director of Scientific Affairs & Research
CLL Society

















